Study on Turmeric Extract Curcumin and Kidneys
Curcumin has a long history and has been used as a medicine for thousands of years in traditional Chinese medicine (Li Mengtao et al. 2010) and Ayurvedic medicine (Goel et al. 2008). As a traditional Chinese medicine extract, curcumin has few side effects at reasonable drug concentrations and has a broad-spectrum therapeutic effect on diseases. It provides new clinical treatment ideas for malignant tumors, chronic diseases and poor surgical prognoses in the cardiovascular, respiratory, nervous, urinary and digestive systems. This paper reviews the latest research progress on the prominent anti-tumor effect of curcumin and its molecular mechanism of action on diseases of different systems.
1. Structure and function of curcumin
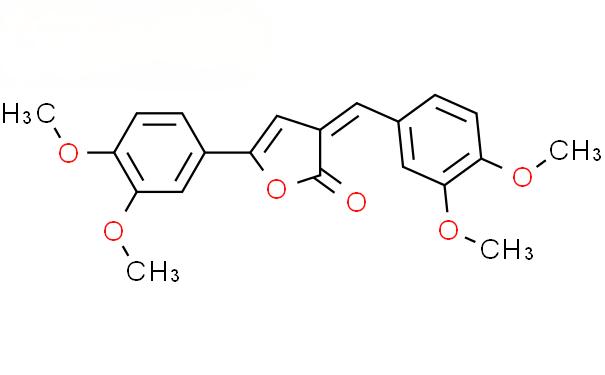
The rhizome of the traditional Chinese medicine turmeric can extract 1% to 6% curcuminoid compounds. Curcumin accounts for 60% to 70% of curcuminoid analogues and is the most biologically active component. The IUPAC name of curcumin is (1E,6E-)1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, with a molecular weight of 368.38 and a molecular formula of C21H20O6. Under different chemical environments with different pH values, the two keto groups on the carbon chain with seven carbon atoms in curcumin can undergo a double keto-enol interconversion, so the chemical structure of curcumin is not stable under physiological pH conditions in the human body.
The main functions of curcumin are reflected in its functional groups: phenolic groups and diketone structures. These two active functional groups mediate the hydrogen-donating reaction, Michael addition reaction, and a series of hydrolytic and enzymatic reactions of curcumin. The phenolic hydroxyl group in the structure of curcumin is most likely to undergo a hydrogen-donating reaction, and is then metabolized into a phenoxy group, which exerts antioxidant properties and scavenges reactive oxygen species (ROS), which are composed of molecular oxidants and free radical oxidants. Secondly, curcumin can act as a nucleophile and undergo a Michael addition reaction with a strong electrophile, exerting cytotoxic effects on cancer cells, i.e., antitumor activity (Priyadarsini et al. 2014). In addition, curcumin also has anti-inflammatory, anti-infective, and lipid metabolism-regulating functions.
2. Molecular mechanism of curcumin in anti-tumor
Today, malignant tumors are increasingly threatening human health, and the incidence and mortality of cancer in China are also on the rise year by year (Wu et al. 2019). The current common treatments for malignant tumors include surgery, radiotherapy, and chemotherapy. However, these common treatment methods have poor prognoses and high recurrence rates. Even drug-targeted therapies (Seebacher et al. 2019) or immunotherapy (Doroshow et al. 2019) have limitations such as gradually increasing drug resistance and severe side effects.
Therefore, it is particularly important to find a method that is superior to traditional treatments for malignant tumors. In recent years, it has been reported that curcumin can inhibit the abnormal proliferation, local invasion, metastasis, and invasion of tumor cells, thereby alleviating the occurrence and development of malignant tumors in affected tissues and organs. Although the pathogenesis and clinical manifestations of malignant tumors in different systems are different or not completely consistent, curcumin has a broad-spectrum inhibitory effect on malignant tumors with extremely high incidence. According to 2018 statistics, the top five cancers in the Chinese cancer spectrum in terms of diagnosis rate are lung cancer, gastric cancer, colorectal cancer, liver cancer, and breast cancer (Feng et al. 2019). Current research shows that curcumin happens to have significant therapeutic effects on these five cancers.
(1) The effect and mechanism of curcumin on lung cancer
Lung cancer is the most representative cancer of the respiratory system. Lung cancer includes small cell carcinoma and non-small cell carcinoma, of which 80% are non-small cell lung cancer (NSCLC) (Evans et al. 2013). The therapeutic effect of curcumin on lung cancer involves multiple mechanisms, including the regulation of the Wnt/β-catenin signaling pathway, which is related to life activities such as cell growth, apoptosis, migration, and differentiation; the signal transducer and activator of transcription 3 (STAT3) signaling pathway, which is abnormally expressed in cancer; and microRNAs and nuclear factor eryth-2-related factor 2 (NRF2), which regulate biological mechanisms such as cell growth. STAT3) signal pathway; micro-RNA (miRNA) and nuclear factor erythroid 2-related factor 2 (Nrf2) signal pathways that regulate biological mechanisms such as cell growth [1].
Cell experiments on clinical human lung tissue samples confirmed that curcumin may significantly reduce the proliferation and migration of NSCLC cells by inhibiting the TLR4/MyD88-EGFR signaling pathway, thereby inhibiting activin-1 and suppressing epithelial mesenchymal transition [2]. However, most current research tends to focus on the combination of curcumin with targeted therapies for some drug-resistant cancers.
Chen et al. [3] found in experiments with gefitinib-resistant NSCLC cells that the combination of curcumin and gefitinib can enhance the efficacy of gefitinib and overcome gefitinib resistance in NSCLC patients with wild-type epidermal growth factor receptors (EGFR) and/or KRAS mutations. The specific mechanism may be related to the degradation of epidermal growth factor receptor protein induced by the proteasome. Bland et al. [4] found through cell experiments that curcumin and its derivatives RL66 and RL118 have inhibitory effects on anaplastic lymphoma kinase-positive lung adenocarcinoma in NSCLC, and that they act on independent targets with no cross-resistance to crizotinib.
(2) Effects and mechanisms on gastric cancer
Experiments with different gastric cancer cell lines and a series of gastric cancer animal model experiments have confirmed that curcumin and its analogues have antioxidant stress and chemosensitization effects, and can inhibit the invasion, proliferation and metastasis of gastric cancer cells through multiple signal pathways in mitochondrial-dependent or non-dependent pathways (Barati et al. 2019). Experiments with gastric cancer cells (BGC-823) and a mouse xenograft gastric tumor model found that the curcumin derivative L6H4 can inhibit the proliferation and invasion of gastric cancer cells BGC-823 both in vitro and in vivo (Mu et al. 2019). Also using cell and mouse tumor model experiments, researchers conducted related research on the curcumin derivative WZ35 and found that WZ35 can inhibit glycolysis by inducing ROS production and downregulating Yes-associated protein (YAP), a downstream effector molecule of the Hippo signaling pathway, and activating c-Jun N-terminal kinase (JNK), thereby inhibiting gastric cancer cell proliferation and promoting apoptosis [5].
Recent research results show that the combination of curcumin and its analogues with chemotherapy can significantly enhance the chemotherapy efficacy of gastric cancer by activating nuclear factor-κB (nuclear factor, NF-κB) and upregulating the level of apoptosis, thereby upregulating the sensitivity of gastric cancer cells to chemotherapeutic drugs [6]. In addition, Kim et al. [7] reported that curcumin can inhibit the formation of benzo[a]pyrene-induced DNA adducts by inhibiting the expression of phase I metabolic enzymes in the stomach of rats, thereby inhibiting DNA damage and thus inhibiting benzo[a]pyrene-induced gastric carcinogenesis.
(3) Effects and mechanisms on colorectal, liver and breast cancers
In addition to the most representative lung cancer and gastric cancer, for colorectal cancer (colorectal cancer, CRC), human colorectal cancer cell lines and CRC animal model experiments have demonstrated the in vivo and in vitro anticancer properties of curcumin in CRC (Pricci et al. 2020); for liver cancer (hepatic cellular cancer, HCC), in cell experiments, curcumin may inhibit the stability of hypoxia-inducible factor-1α (HIF-1α) by upregulating Nrf2 and glutathione, thereby inducing ROS scavenging, which in turn inhibits the expression of connective tissue growth factor (CTGF), ultimately exhibiting a protective effect on HCC (Shao et al. 2019); for breast cancer, in cell experiments and xenograft mouse models and lung metastasis models, the curcumin derivative WZ35 can inhibit breast cancer cell growth through the ROS-YAP-JNK signaling pathway. (Wang et al. 2019).
3. The role of curcumin in various systems
(1) Curcumin participates in the protective effect of the cardiovascular system
Atherosclerosis caused by dyslipidemia and exogenous induced ischemia-reperfusion injury are common diseases of the cardiovascular system. Curcumin has the effects of lowering blood lipids and resisting oxidative stress, suggesting its potential clinical application value in cardiovascular system diseases. In cell and mouse experiments, the protective effect of curcumin on cardiomyocytes may be achieved by downregulating the Notch pathway and reducing intracellular ROS levels[8]. In addition, Guan et al. [9] confirmed that curcumin can reduce the decrease in H9C2 cell viability and promote apoptosis induced by palmitic acid through activation of the endoplasmic reticulum stress pathway. Animal experiments have found that curcumin can also regulate blood pressure. Curcumin can lower blood pressure by inhibiting angiotensin-converting enzyme and angiotensin II levels in the brain (Kim et al. 2019).
Clinical trials have shown that curcumin can lower homocysteine and increase HDL concentrations in young obese men, thereby improving the risk factors for obesity-related cardiovascular disease (Campbell et al. 2019). Clinical trials have also shown that curcumin can significantly improve the LDL cholesterol, very low density lipoprotein, and triglyceride levels of atherosclerotic patients when taken orally. This may be related to the fact that curcumin can participate in lipid metabolism, lipid oxidation, and oxidative stress processes (Ding Xiujuan et al. 2020). Coronary microembolism and restenosis are common complications of percutaneous coronary intervention. Curcumin may inhibit myocardial cell apoptosis and inflammatory response by suppressing the TLR4/MyD88/NF-κB signaling pathway and downregulating the expression of inflammatory factors tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), and ultimately protects against myocardial injury induced by coronary artery microembolism in rats [10]. In addition, experiments using a rat carotid artery model have confirmed that curcumin can also prevent neointimal hyperplasia and inhibit postoperative vascular restenosis (Akhlaghi et al. 2019).
(2) The protective effect of curcumin and its analogues on acute lung injury
Respiratory diseases such as acute lung injury (ALI) and acute respiratory distress syndrome are often accompanied by an inflammatory storm, oxidative stress, and even bacterial infection. Curcumin inhibits all three pathological processes. Chai et al. [11] found in a mouse ALI model induced by cecal ligation and puncture that curcumin can regulate the development of immature CD4+ T cells and their differentiation into CD4+CD25+FOXP3+ Tregs, and ultimately exert anti-apoptotic, anti-inflammatory and immunomodulatory effects by promoting the transformation of macrophages. For the treatment of ALI, in addition to the conventional oral or intraperitoneal administration of curcumin, inhalation of the drug in combination with a carrier can also be used. Kim et al. [12] combined curcumin with a heme oxygenase-1 gene plasmid with anti-inflammatory activity, and used inhalation to administer the mixture to an ALI mouse model via a poly-amidoamine (PamChol) carrier linked to cholesterol. This achieved an anti-inflammatory effect superior to that of conventional administration.
In addition, Qian et al. [13] found in an in vivo anti-inflammatory activity evaluation of ALI in mice that most synthetic dicarbonyl curcumin analogues represented by DACs 5a27 and 5a28 can effectively inhibit lipopolysaccharide-induced TNF-α and IL-6 levels, block the mitogen-activated protein kinase (MAPK) signaling pathway, activate NF-κB, and exert an anti-inflammatory effect against ALI. In addition, cell and animal experiments have found that [14] curcumin analog C66 combined with JNK inhibitor SP600125 can inhibit the expression of inflammatory cytokines by inhibiting the phosphorylation of JNK, that is, inhibiting the MAPK pathway, ultimately relieving the inflammatory response and reducing acute lung injury induced by intratracheal instillation of lipopolysaccharide. A series of toxic effects caused by ingestion of paraquat, including oxidative stress, often severely damage lung function, leading to ALI. Experiments have found that curcumin incubation significantly reduces the ROS level and apoptosis rate of paraquat-pretreated normal lung fibroblasts (WI-38VA13), which may be related to the inhibition of the TXNIP/NLRP3 inflammatory axis [15].
(3) Biological effects of curcumin on neurological diseases
Curcumin is the main component of a compound traditional Chinese medicine for the treatment of depression (Li Chuanpeng et al. 2020), and it is now generally believed that this therapeutic mechanism may be related to the regulation of the hypothalamus-pituitary-adrenal axis (HPA axis), HPA axis) regulation; it is associated with dysregulation of monoamine neurotransmitters such as dopamine and serotonin, as well as levels of brain-derived neurotrophic factor; it is also associated with inflammation and immune pathway dysregulation [16]. In a meta-analysis based on clinical trials, researchers gave curcumin a certain degree of recognition as an adjuvant treatment for depression. However, these meta-analyses are also limited by the number and quality of the samples, and the clinical efficacy of curcumin needs to be further evaluated (Fusar-Poli et al. 2020).
In view of the above therapeutic effect of curcumin on neuropsychiatric diseases, current clinical trials have focused on studying the effect of curcumin on the relief of neurological complications, with the ultimate goal of achieving a high cure rate. One clinical trial confirmed that a continuous 8-week nano-curcumin supplement was superior to the placebo group in reducing depression and anxiety scores in patients with diabetic peripheral neuropathy (Asadi et al. 2019). In terms of the molecular mechanism of curcumin intervention in neuroinflammation, Zhang et al. [17] first discovered that curcumin can promote the phenotypic transformation of microglia through the TREM2/TLR4/NF-κB signaling pathway to reduce neuroinflammation. Depressive mood can lead to a decrease in pain threshold, and curcumin can alleviate depressive disorders and postoperative pain, but the relationship and mechanism between the two have not yet been elucidated. In terms of neurodegenerative diseases, curcumin can inhibit oxidative stress, neuroinflammation and apoptosis through the Nrf-2/TLR4/RAGE signaling pathway in ethanol-induced in vivo and in vitro experiments in mice, thereby improving neurodegeneration and cognitive dysfunction [18]. Curcumin can also chelate with heavy metals to inhibit the oxidative stress induced by lead in the cerebellum of rats, thereby improving motor and cognitive dysfunction and neuronal function [19].
(4) Curcumin's alleviating effect on kidney tissue damage
Curcumin can significantly reduce the kidney tissue damage induced by lipopolysaccharide in a mouse model of acute kidney injury and inhibit the expression of lncRNA PVT1. Moreover, this result may be related to curcumin's inhibition of the PVT1/JNK/NF-κB signaling pathway [20]. Huang et al. [21] found that curcumin can also alleviate the nephrotoxicity of cisplatin-induced acute kidney injury in mice through the miR-81a/PTEN axis. In addition, cell and mouse model experiments have confirmed that curcumin can reduce septic acute kidney injury by inhibiting the NF-κB and JAK2/STAT3 signaling pathways (Zhu et al. 2020). In addition, experiments with lipopolysaccharide-induced RAW264.7 cells found that curcumin can inhibit macrophage-induced type C lectin, thereby maintaining the phenotype of M1 macrophages and ultimately reducing acute kidney injury [22].
Curcumin also has a beneficial effect on chronic kidney disease. Curcumin can down-regulate uric acid levels in a mouse model of hyperuricemia induced by potassium oxonate, further inhibit NLRP3 inflammasome activation by uric acid and IL-1β levels, and ultimately inhibit chronic renal inflammation caused by hyperuricemia [23]. Li et al. [24] found in an experimental mouse model of kidney stones induced by glyoxalate that curcumin can effectively reduce the deposition of CaOx crystals and the associated damage to kidney tissue through multiple mechanisms, including antioxidant stress, anti-inflammation, anti-apoptosis, anti-fibrosis, and inhibition of the autophagy mechanism. reduce the deposition of CaOx crystals and the accompanying damage to kidney tissue. However, in terms of research into the relevant molecular mechanisms, this experiment has only preliminarily discovered that curcumin exerts its anti-stone function at least partially through the Nrf2 signaling pathway.
(5) Curcumin's effect on alleviating liver tissue damage
The mechanism by which curcumin alleviates the pathological and physiological damage to digestive organs (such as the liver and pancreas) is complex. The classical pathway is related to the immune pathway mediated by inflammatory cytokines and oxidative stress. The combined administration of curcumin and Erzi Wan can inhibit the levels of inflammatory cytokines (IL-4 and TNF-α) induced by Concanavalin A in mice with immune hepatitis, downregulate their serum levels of alanine aminotransferase and aspartate aminotransferase, thereby reducing inflammation and alleviating pathological liver damage. The specific mechanism may be related to the inhibition of the Nrf-2 signaling pathway [25].
Luo et al. [26] conducted a more detailed study of the molecular pathways in which Nrf-2 is located. They found that the two hydrolyzed metabolites of curcumin, tetrahydrocurcumin and octahydrocurcumin, can inhibit the activity and expression of CYP2E and activating the Kelch-like ECH-associated protein 1 (Kelch-like ECH-associ- ated protein 1 , Keap1) -nuclear factor E2-related factor 2 (nu- clear factor-E2-related factor 2 , Nrf2) pathway, improving acetaminophen-induced liver damage in mice. Curcumin can also alleviate non-alcoholic fatty liver disease in mice caused by a high-fat diet by improving intestinal barrier function, reducing endotoxins, Toll-like receptor-4 (TLR4) and NF-κB inflammation [27]. For the treatment of non-alcoholic liver steatosis, unlike Luo, Gheibi et al. [28] used curcumin in combination with ursodeoxycholic acid, and obtained similar improvement results as curcumin alone through experiments on rats. However, this combination therapy was more effective than curcumin or ursodeoxycholic acid alone.
4. Conclusion and outlook
Curcumin exerts a positive biological effect on various human systems and organs through the mediation of multiple molecular pathways. Curcumin can regulate blood lipid levels, inhibit oxidative stress, and regulate blood pressure, thereby exerting protective effects such as anti-apoptosis and anti-inflammation on cardiomyocytes and blood vessels. In the treatment of respiratory diseases represented by ALI, curcumin and its analogues also play an immune-modulating role in addition to anti-inflammatory and anti-oxidative stress effects. For neurological diseases, curcumin is mainly used as an adjuvant therapy to relieve nerve and mental disorders.
In addition, curcumin also relieves the corresponding damage to kidney and liver tissues in urinary and digestive system diseases. Therefore, curcumin, which has many pharmacological properties, has good research and application prospects. However, the oral bioavailability of curcumin is relatively low. Therefore, the current research and development of drugs that modify the structure of curcumin, the activity screening of curcumin analogues, derivatives, and metabolites with similar functions, and the study of their mechanisms of action are of high research value. Although cell experiments, animal experiments and clinical trials have confirmed that the mechanism of action of curcumin is related to the interaction of multiple pathways, the specific molecular mechanism of curcumin in each signaling pathway has not yet been elucidated in detail. In addition, clinical pharmacokinetic concentration screening experiments with different gradients still need to be carried out for a specific disease in a certain system and for a certain age range of patients in order to determine the safe and effective curcumin use concentration range. This study provides a theoretical basis for the future combination use of curcumin and as a biological health care drug.

Referenece:
[1]Ashrafizadeh M , Najafi M , Makvandi P , et al. Versatile role of curcumin and its derivatives in lung cancer thera- py. J Cell Physiol , 2020 , 235 : 9241~9268.
[2]Zhang L , Tao X , Fu Q , et al. Curcumin inhibits cell proliferation and migration in NSCLC through a syner- gistic effect on the TLR4/MyD88and EGFR pathways . Oncol Rep, 2019 , 42 : 1843~1855.
[3]Chen P , Huang HP , Wang Y , et al. Curcumin over- come primary gefitinib resistance in non-small-cell lung cancer cells through inducing autophagy-related cell death. J Exp Clin Cancer Res , 2019 , 38 : 254.
[4]Bland AR , Bower RL , Nimick M , Hawkins BC , Rosen- gren RJ , Ashton JC. Cytotoxicity of curcumin deriva- tives in ALK positive non-small cell lung cancer. Eur J Pharmacol , 2019 , 865 : 172749.
[5]Chen T , Zhao L , Chen S , et al. The curcumin analogue WZ35affects glycolysis inhibition of gastric cancer cells through ROS-YAP-JNK pathway. Food Chem Toxicol , 2020 , 137 : 111131.
[6] Zhou L , Li Y , He W , et al. Mutual antagonism of Wilms'tumor 1and β-catenin dictates podocyte health and disease. J Am Soc Nephrol , 2015 , 26 : 677.
[7]Kim KS , Kim NY , Son JY , et al. Curcumin ameliorates benzo[a] pyrene-induced DNA damages in stomach tis- sues of sprague-dawley rats . Int J Mol Sci , 2019 , 20 : 5533.
[8]Zhu P , Yang M , He H , et al. Curcumin attenuates hypoxi- a/reoxygenation-induced cardiomyocyte injury by down- regulating Notch signaling. Mol Med Rep , 2019 , 20 : 1541~1550.
[9]Guan G , Lei L , Lv Q , et al. Curcumin attenuates pal- mitic acid-induced cell apoptosis by inhibiting endoplas- mic reticulum stress in H9C2cardiomyocytes . Hum Exp Toxicol , 2019 , 38 : 655~664.
[10] Liu Y , Liu Y , Huang X , et al. Protective effects and mechanism of curcumin on myocardial injury induced by coronary microembolization. J Cell Biochem , 2019 , 120 : 5695~5703.
[11]Chai YS , Chen YQ , Lin SH , et al. Curcumin regulates the differentiation of naïve CD4+ T cells and activates IL-10immune modulation against acute lung injury in mice. Biomed Pharmacother , 2020 , 125 : 109946.
[12] Kim G , Piao C , Oh J , Lee M. Combined delivery of curcumin and the heme oxygenase-1 gene using choles- terol-conjugated polyamidoamine for anti-inflammatory therapy in acute lung injury. Phytomedicine , 2019 , 56 : 165~174.
[13]Qian J , Chen X , Shu S , et al. Design and synthesis no- vel di-carbonyl analogs of curcumin (DACs) act as po- tent anti-inflammatory agents against LPS-induced acute lung injury (ALI) . Eur J Med Chem , 2019 , 167 : 414 ~ 425.
[14]Xiao Z , Xu F , Zhu X , et al. Inhibition of JNK phospho- rylation by curcumin analog C66 protects LPS-induced acute lung injury. Drug Des Devel Ther , 2019 , 13 : 4161~4171.
[15]Ren Y , Yang Z , Sun Z , et al. Curcumin relieves pa- raquat-induced lung injury through inhibiting the thiore- doxin interacting protein/NLR pyrin domain containing 3-mediated inflammatory pathway. Mol Med Rep , 2019 , 20 : 5032~5040.
[16]Zhang Y , Li L , Zhang J . Curcumin in antidepressant treatments : An overview of potential mechanisms , pre- clinical/clinical trials and ongoing challenges . Basic Clin Pharmacol Toxicol , 2020 , 127 : 243~253.
[17]Zhang J , Zheng Y , Luo Y , et al. Curcumin inhibits LPS-induced neuroinflammation by promoting microgli- al M2 polarization via TREM2/ TLR4/ NF-κB path- ways in BV2cells . Mol Immunol , 2019 , 116 : 29~37.
[18]Ikram M , Saeed K , Khan A , et al. Natural dietary sup-plementation of curcumin protects mice brains against ethanol-induced oxidative stress-mediated neurodegener- ation and memory impairment via Nrf2/TLR4/RAGE signaling. Nutrients , 2019 , 11 : 108.
[19] Abubakar K , Mailafiya MM , Danmaigoro A , et al. Curcumin attenuates lead-induced cerebellar toxicity in rats via chelating activity and inhibition of oxidative stress . Biomolecules , 2019 , 9 : 453.
[20]Huang W , Li X , Wang D , et al. Curcumin reduces LPS- induced septic acute kidney injury through suppression of lncRNA PVT1in mice. Life Sci , 2020 , 254 : 117340.
[21] Huang SJ , Huang J , Yan YB , et al. The renoprotective effect of curcumin against cisplatin-induced acute kidney injury in mice : involvement of miR-181a/PTEN axis . Ren Fail , 2020 , 42 : 350~357.
[22]Tan RZ , Liu J , Zhang YY , et al. Curcumin relieved cisplatin-induced kidney inflammation through inhibiting Mincle-maintained M1 macrophage phenotype. Phyto- medicine , 2019 , 52 : 284~294.
[23]Chen Y , Li C , Duan S , et al. Curcumin attenuates potas- sium oxonate-induced hyperuricemia and kidney inflam- mation in mice. Biomed Pharmacother , 2019 , 118 : 109195.
[24]Li Y , Zhang J , Liu H , et al. Curcumin ameliorates glyoxylate-induced calcium oxalate deposition and renal inj uries in mice. Phytomedicine , 2019 , 61 : 152861.
[25] Li Y, Shao Y, Tang H, et al. Protective effect of curcumin combined with Erzi Wan on kidney bean protein A-induced immune hepatitis in mice. Shizhen National Medicine National Medicine, 2020, 31: 769–773.
[26]Luo DD , Chen JF , Liu JJ , et al. Tetrahydrocurcumin and octahydrocurcumin , the primary and final hydrogenated metabolites of curcumin , possess superior he- patic-protective effect against acetaminophen-induced liver injury : Role of CYP2E1and Keap1-Nrf2 pathway. Food Chem Toxicol , 2019 , 123 : 349~362.
[27] Feng D , Zou J , Su D , et al. Curcumin prevents high-fat diet-induced hepatic steatosis in ApoE-/ -mice by improving intestinal barrier function and reducing endotoxin and liver TLR4/NF-κB inflammation. Nutr Metab (Lond) ,2019 , 16 : 79.
[28] Gheibi S , Ghaleh HEG , Motlagh BM , et al. Therapeu- tic effects of curcumin and ursodexycholic acid on non- alcoholic fatty liver disease. Biomed Pharmacother , 2019 , 115 : 108938.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese