Study on Curcumin Powder Microencapsulation Technology
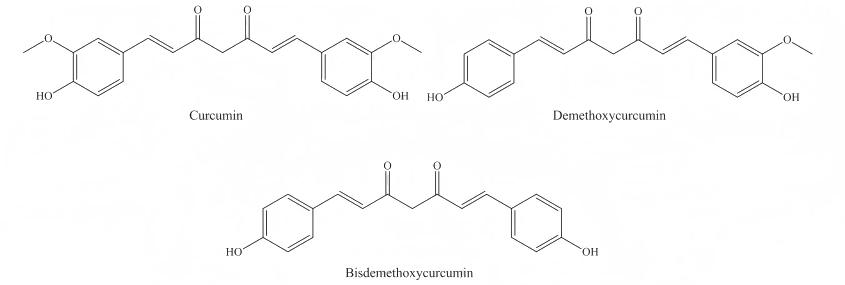
Curcuminoids are a class of compounds purified from the rhizome part of the turmeric plant Curcuma longa. They mainly include curcumin (Cur), demethoxycurcumin (DMC) and bis-demethoxycurcumin (Bis-DMC), each accounting for 60% to 75%, 10% to 27%, and 5% to 15%, respectively. As an active substance, curcumin has various effects such as antioxidant [1-4], anti-inflammatory [5-9], and anticancer [10-15]; moreover, in the long history of human development, there have been medicinal records of turmeric for a long time. For example, the Indian medicine book Ayurveda called it the “spice of life”, and China's great pharmacology classic Compendium of Materia Medica also recorded that turmeric can be used to treat heart pain, abdominal pain, sores, etc. This shows that curcumin has broad application prospects in the fields of food, medicine, and daily chemicals.
However, curcumin is easily degraded by light, heat, oxygen, acid and alkali, and these problems limit its application and scope of use. A microcapsule is a spherical particle with a diameter of 50 nm to 2 mm that contains a core material, or the core material is dispersed in a wall material matrix. At present, microencapsulation technology has been widely used in the delivery systems of active substances, which can improve the light, heat, oxygen, and acid-base stability of active substances, and also has a certain controlled-release effect during in vivo and in vitro digestion processes [16]. Therefore, curcumin microencapsulation technology is a good way to improve the application effect of curcumin and broaden its scope of application. Therefore, this paper describes the physicochemical properties, in vivo metabolic processes and biological activities of curcumin, and focuses on a review of the microencapsulation technology of curcumin.

1 Overview of curcumin
1.1 Structure and physicochemical properties of curcumin
Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] is a bis-ferulic acid compound with the molecular formula C21H20O6 and molecular weight 368.37 g/mol. Its melting point is 179–183 °C, and its chemical structure is a β-diketone with an o-methoxyphenol group attached to each end (see Figure 1). The transfer of hydrogen atoms on the β-diketone gives curcumin a keto-enol tautomer (see Figure 2). Under slightly acidic and neutral conditions, curcumin exists in the keto form, while under alkaline conditions, it exists in the enol form [17-19].
Curcumin is an orange-yellow crystal that has been used in food processing as a natural pigment. It is red when the pH is <1, yellow when the pH is 1–7, and orange-red when the pH is >7.5 [20]. Curcumin is very insoluble in water, with a solubility of about 11 ng/mL [21]. Its low solubility is mainly due to its highly hydrophobic structure and crystalline nature. When curcumin exists in a crystalline state, it forms intermolecular and intramolecular hydrogen bonds [22], which inhibit the solubility of curcumin in water. However, it is easily soluble in organic solvents such as methanol, ethanol, acetone and dimethyl sulfoxide.
Turmeric extract is unstable; it begins to degrade at 70 °C, and about 32% of it degrades after 20 minutes at 70 °C [23]. It is also sensitive to light, and under light conditions, the β-diketone loses two hydrogen atoms to form small molecule phenolic compounds such as vanillic acid, vanillin, ferulic acid, ferulic aldehyde, etc. [24], or undergo demethoxylation and keto-enol isomerization to form by-products such as diketones and methanol, acetate, etc. [25]. At present, most scholars believe that curcumin is extremely unstable under alkaline conditions, decomposing to produce substances such as ferulic acid, ferulic acid methyl ester, and vanillin, and turns yellow or brown. It is more stable under acidic and neutral conditions [20, 26-27], which may be related to its conjugated diene structure [26]. However, some scholars believe that curcumin is more unstable under acidic conditions, and its degradation rate is about 20 times higher than that under neutral or alkaline conditions [28].
1.2 Biological activity of curcumin
1.2.1 Antioxidant
Curcumin is a phenolic chain-breaking antioxidant. Its antioxidant effect is mainly achieved by scavenging reactive oxygen species (ROS) and enhancing the activity of antioxidant enzymes and phase II metabolic enzymes as an inducer of antioxidant pathways [29].
Regarding the activity of curcumin in scavenging ROS, some scholars believe that it is due to the dissociation of hydrogen on the phenolic group [3, 30-32], while others believe that it is due to the dissociation of hydrogen on the central methylene group of the β-diketone structure [33]. In Guo et al. [1], compared with the blank control, curcumin treatment of human corneal endothelial cells after induced oxidative stress, cell viability increased, and intracellular ROS production decreased, which was mainly due to curcumin significantly enhanced the expression of nuclear transcription factors (NF-κB) in the cell, as well as Keap1/ Nrf2 / ARE pathway superoxide dismutase 1 and heme oxygenase 1 production, thereby enhancing the antioxidant capacity of human corneal endothelial cells. Deng et al. [34] found that curcumin and its analogues can effectively inhibit free radical-induced oxidative hemolysis of red blood cells. Momeni et al. [4] evaluated the protective effect of curcumin against the nephrotoxicity of sodium arsenite. Curcumin, as an antioxidant, can slow down or prevent the adverse effects of sodium arsenite on the glomerulus and proximal tubules in kidney tissue and the antioxidant capacity of the serum. In the study by Rai et al. [2], curcumin was comparable to antioxidant drugs in the treatment of submucosal fibrosis of the oral cavity, and it significantly improved the patient's mouth opening, the burning sensation when eating spicy food, and the tongue papillae.
1.2.2 Anti-inflammatory
Inflammation is the body's defensive response to stimuli. It is an important part of the immune system's function of maintaining homeostasis, and is associated with many chronic diseases, such as obesity, diabetes, chronic obstructive pulmonary disease and atherosclerosis [7]. Curcumin can effectively inhibit inflammation by inhibiting inflammatory mediators and nuclear transcription factors (NF-κB) and enhancing the action of glucocorticoids [35]. For example, curcumin can reduce inflammation by upregulating SIRT1 to prevent the activation of NLRP3 inflammasomes, thereby protecting against acute pneumonia [9]. In an in vitro inflammation model induced by tumor necrosis factor α (TNF-α) (human placenta, visceral adipose tissue, and subcutaneous adipose tissue), curcumin significantly inhibited inflammatory mediators (interleukins 1A, 1B, and 6) and promoted the expression of anti-inflammatory cytokines interleukins 4 and 3. Therefore, curcumin is expected to be used as a therapeutic intervention for pro-inflammatory pregnancy complications [9]. Curcumin reduces the inflammatory response by down-regulating the levels of inflammatory mediators (TNF-α, interleukin-1β and 17, and transforming growth factor-β) in the joints of rats with collagenous arthritis, inhibits the expression of cyclooxygenase (COX-2), and also induces macrophage apoptosis to exert a therapeutic effect on collagenous arthritis [6].
1.2.3 Anti-tumor
Curcumin exerts its anti-tumor effect mainly by inducing apoptosis in tumor cells, inhibiting tumor invasion and metastasis, and reversing drug resistance in tumor cells [36]. For example, curcumin enhances the expression of miR-99a in retinoblastoma to block the JAK/STAT pathway, thereby inhibiting cell malignancy [10]. Curcumin inhibits the proliferation of glioblastoma by blocking the AKT/MTOR pathway [11]. Curcumin inhibits the phosphorylation of protein kinases (extracellular regulated protein kinases, ERK) in human colon cancer cells, which leads to the suppression of the downstream ERK signals c-MYC and cyclin D1. The proportion of G0/G1 cells increases, which in turn blocks the cell cycle of colon cancer cells and induces apoptosis [13]. In addition, in Xu et al. [12], CD44 is one of the common markers on the surface of colon cancer cells, and curcumin can down-regulate the expression of CD44 and inhibit the proliferation, migration and tumor sphere formation of colon cancer cells. Therefore, curcumin may be a colon cancer adjuvant therapy drug that targets CD44.
1.2.4 Other biological activities
In addition to the above-mentioned antioxidant, anti-inflammatory and anti-tumor functions, curcumin has a variety of other functional activities. For example, curcumin can significantly inhibit the growth of Helicobacter pylori at concentrations above 200 μmol/L [37]; curcumin can improve metabolic disorders in diabetes, effectively regulate blood sugar and blood lipids, improve β-cell function, lower insulin resistance, thereby exerting a hypoglycemic effect [38]; curcumin can also bind to ligands in fat cells, exerting an inhibitory effect on adipocyte differentiation and has the potential to prevent obesity [39]. From this it can be seen that curcumin is a bioactive substance with great development potential.
1.3 Absorption, metabolism and bioavailability of curcumin
1.3.1 Absorption and metabolism
Since 1978, when Holder et al. [40] first studied and reported the metabolites of curcumin in rats, many researchers at home and abroad have conducted a series of in vitro and in vivo metabolic studies on curcumin.
The main metabolic pathways of curcumin in vivo include phase I reduction metabolism and phase II conjugation metabolism [41] (see Figure 3), as well as degradation, auto-oxidation and catalytic oxidation of curcumin. Curcumin I-phase reductive metabolism is a NADPH-dependent stepwise hydrogenation process of the four double bonds in the β-diketone structure [42]. This process is mainly catalyzed by cytochrome P450 and alcohol dehydrogenase in the cytoplasm of liver and small intestine cells [43]. The main products include dihydrocurcumin, tetrahydrocurcumin (Tetrahydrocurcumin, THC), hexahydrocurcumin and octahydrocurcumin [44]. The combined metabolism of curcumin II refers to the process in which curcumin or its phase I reduced metabolites are glucuronidated or sulfated under the catalysis of glucuronidases or sulfotransferases, with glucuronidation being the main process [45].
The uridinediphosphate glucuronosyltransferase (UGT) adds the glycosyl group of uridine-5'-diphosphate glucuronic acid to curcumin or its phase I metabolites, enhancing the water solubility of these substances and making them more easily excreted from the body in the urine [46]; UGT is mainly distributed in the endoplasmic reticulum of cells in organs such as the liver, intestines, and kidneys. UGT in the liver (UGT1A1, UGT1A9) mainly catalyzes the production of phenolic hydroxyl glucuronide conjugates and a small amount of alcohol hydroxyl glucuronide conjugates from curcuminoids.
Intestinal UGTs can only catalyze the production of phenolic hydroxyl glucuronic acid conjugates from curcuminoids, such as UGT1A8 and UGT1A10 [47]. In addition, UGT1A7, UGT1A8, and UGT1A10 exhibit high activity towards hexahydrocurcumin, but UGT1A7, UGT1A8, and UGT1A10 are inactive or have low activity in the liver, but are specifically expressed in the gastrointestinal tract [48-49]. It can be seen that, in addition to the liver, the gastrointestinal tract may be the main organ for the glucuronidation of curcumin. Sulfotransferases (SULTs) can transfer the sulfonyl group in SO3- to substrates containing hydroxyl or amino groups to form polar products that are more easily removed from the body [50]. The small intestine is the main tissue in the body for the sulfation of curcumin. SULT1A3 can mediate the sulfonation of curcumin and demethoxycurcumin, SULT1B1 only mediates the sulfonation of curcumin, and SULT1C4 catalyzes all three curcuminoids [51].

In addition to the above metabolic processes, intestinal microorganisms also play an important role in the metabolism of curcumin. For example, the human intestinal bacterium Blautia sp. MRG-PMF1 can convert curcumin to DMC and Bis-DMC, or convert DMC to Bis-DMC [52]; in a human fecal model, the three curcuminoids (Cur, DMC, Bis-DMC) were degraded, with degradation rates of 24%, 61% and 87%, respectively. THC, Dihydroferulic acid (DFA) and 1-(4-hydroxy-3-methoxyphenyl)-2-propanol were identified by ultra-high performance liquid chromatography and mass spectrometry [53]. Curcumin produces different metabolites under different intestinal flora. It has been reported that curcumin can undergo various transformations such as demethylation, reduction, hydroxylation, acetylation, and methylation under the action of intestinal flora [52-55]. In addition, many scholars have proposed that there is a mutual interaction between curcumin and intestinal flora, that is, curcumin can produce different metabolites during the biotransformation of intestinal microorganisms, and in turn, curcumin and its metabolites have a regulatory effect on the balance of intestinal flora [56-59]; and the balance of human flora has an important impact on health. Therefore, this can be used to explain the contradiction between the low bioavailability of curcumin and its widely reported beneficial effects.
1.3.2 Bioavailability
After oral administration, curcumin is mainly excreted in the feces in the form of the parent drug. Wahlstrom and Blennow [60] orally administered curcumin (1 g/kg) to SD rats, and after 72 h, approximately 75% of the curcumin was excreted from the rats in the feces, and negligible curcumin was detected in the urine (< 0.0006%). Curcumin is rapidly metabolized in the liver or blood. In liver cells or liver microsomal suspensions, 90% of curcumin is metabolized within 30 minutes [60]; after intraperitoneal injection of curcumin (100 mg/kg), the peak plasma concentration of curcumin is 2.25 μg/mL at 15 minutes [61]; after intravenous injection of curcumin, the plasma concentration of curcumin is about 0.02 μg/mL, and the concentration approaches zero at 60 min [60]. Curcumin has poor absorption and rapid metabolism, which leads to its low bioavailability.
2 Curcumin microencapsulation technology
Microcapsules can effectively enhance the solubility and stability of functional ingredients and improve their bioavailability by encapsulating bioactive substances. According to the internal structure and morphology of the microcapsules, such as single-layer or multi-layer wall material, core material wrapped inside the microcapsule or dispersed in the wall material matrix, spherical or irregular shape, etc. [62], turmeric curcuminoid microcapsules can be broadly divided into the following types (see Figure 4).
2.1 Main methods of curcumin microencapsulation
2.1.1 Spray drying method
The principle of spray drying is to disperse the core material into the wall material solution to form a stable and uniform feed solution. The feed solution is then dispersed into tiny droplets under the action of high-speed compressed air from the atomizer. The water in the droplets evaporates rapidly under the action of the high-temperature airflow in the drying chamber, and the wall material solidifies to form dry microcapsule particles. The spray drying method simultaneously achieves the preparation and drying of microcapsules. It has the characteristics of being simple in process, low in cost, easy to implement industrial production, characteristics of the microencapsulation of heat-sensitive substances [63]. It is the most widely used microencapsulation technology [64] and is also a common method for embedding curcumin.
In the preparation of curcumin microcapsules, spray drying is usually combined with emulsification. The viscosity of the emulsion and the spray drying parameters are the two main factors affecting the quality of curcumin microcapsules. The viscosity of the emulsion is affected by the type of wall material and the core-to-wall ratio. The wall material used for spray drying should have good water solubility, maintain a low viscosity even at high concentrations, be easy to atomize, easy to dehydrate and dry, and have few wall attachments [65]. Carbohydrates and proteins are commonly used. Reducing the core-to-wall ratio, i.e., increasing the proportion of wall material, increases the viscosity of the emulsion.

Meena et al. [66] investigated the effect of the core-wall ratio on the encapsulation of curcumin microcapsules at core-wall ratios of 1:1, 1:2, and 1:3. As the proportion of wall material increased, the encapsulation rate increased in turn, but there was no significant difference in the encapsulation rate under the conditions of 1:2 and 1:3. Moreover, as the solids content increased, the yield of the product showed an upward trend and then a downward trend, which may be due to the increased viscosity of the emulsion and poor droplet atomization [67-68]. Spray drying parameters such as feed rate and spray drying temperature have an impact on the quality of the microcapsules and the curcumin in the microcapsules [69]. If the feed rate is too fast or the spray drying temperature is too low, the particles will not be adequately dried, the moisture content in the microcapsules will increase, curcumin will tend to form crystals, and in a two-fluid nozzle spray dryer, if the core material feed rate is too fast, there will not be enough wall material to coat the core material, and the prepared microcapsules will be too large in size [69].
Curcumin microcapsules prepared by spray drying technology significantly improve the thermal stability of curcumin. After being stored at 70 °C for the same period of time, the degradation rate of curcumin in microcapsules is about 20%, while the degradation rate of unencapsulated curcumin is higher than 90% [70]. After simulated gastric digestion, about 88% of curcumin is still retained in the microcapsules, After simulated intestinal digestion, 86.36% curcumin was released [66]; unencapsulated curcumin was almost completely degraded when placed under a 5 W LED for 12 d [70], while the retention rate of microencapsulated curcumin was 84.154% after 8 weeks of storage [71]. Andrade et al. [72] confirmed that the spray drying process does not affect the functional activity of curcumin.
After spray drying, curcumin in the microcapsules can still significantly reduce the level of TNF-α and still has the potential to treat neurodegenerative diseases. The spray drying process does not affect the biological activity of curcumin, but instead enhances the anticancer activity of curcumin in the microcapsules. This may be due to the fact that the microencapsulation technology improves the solubility of curcumin and increased the uptake of curcumin by cells, thereby increasing its effective concentration and enhancing its activity [73]. Compared with freeze-drying, although the instantaneous high temperature during the spray drying process causes partial degradation of the curcumin in the microcapsules, in the study by D. M. CANO-HIGUITA et al. [71], the retention rate of curcumin in spray-dried microcapsules under the same storage conditions was 84.154%, while the retention rate of curcumin in freeze-dried microcapsules was only 63.832%. This shows that spray drying is a good method for preparing curcumin microcapsules.
2.1.2 Coacervation method
The coacervation method is based on the principle that two polymers with opposite charges, such as protein-polysaccharide, protein-protein, and polysaccharide-polysaccharide, reduce the solubility of their complexes due to charge neutralization, and deposit and encapsulate around the core material to form microcapsules. This method is only suitable for microencapsulation of non-water-soluble functional ingredients. In addition to electrostatic attraction and charge neutralization, non-covalent interactions (such as hydrogen bonding and hydrophobic interactions) also contribute to the formation of microcapsules during the complex coacervation method [74]. Curcumin microcapsules prepared by the complex coacervation method have a high encapsulation rate (see Table 1), which provides good protection for curcumin in light and heat environments, and also has a good slow-release effect on curcumin in in vitro simulated digestion tests. However, the complex coacervation method is affected by factors such as the pH of the system, the concentration between the two polymers, and the temperature, and the conditions are difficult to control and the process is cumbersome. The pH of the system determines the charge of the polymer, and the ratio between the two polymers controls the charge balance during the complexation process [75], which in turn affects the interaction between the polymers, as well as the quality and yield of the microcapsules.
At a pH where both wall material molecules carry the maximum equivalent opposite charge, the interaction between the two wall material molecules is the strongest, the most complexes are formed, and the yield of microcapsules is also the highest [76-77]. In Mohammadian et al. [78], the turbidity of the system was highest at pH = 3, indicating the formation of a large number of whey protein nanofibers-gum arabic complexes, while at higher pH (closer to the isoelectric point of whey protein), the turbidity of the system was much lower than that at pH = 3. An imbalance of electrical charges will result in weak electrostatic interactions between the molecules of the two wall materials and a lower agglomeration yield. In the study by Kavousi et al. [79], when the ratio of cress seed mucilage (CSM) to sodium caseinate was adjusted to 1:2, the number of negative charges carried by CSM was the same as the number of positive charges carried by sodium caseinate, and the turbidity of the system was the highest.
2.1.3 Molecular Encapsulation Method
The molecular encapsulation method, also known as the molecular encapsulation method or the molecular encapsulation method, is a microencapsulation method that occurs at the molecular level. This method mainly uses the intermolecular forces between the core material and the wall material to form molecular microcapsules. This method usually uses cyclodextrins and their derivatives as the wall material. The encapsulation process is a physical process [83] without a chemical reaction, which allows the original properties and functions of the active substance to be retained. There are three main methods for preparing curcumin-cyclodextrin complexes: (1) the saturated aqueous solution method, in which an aqueous solution of cyclodextrin is mixed with an organic solvent solution of curcumin, and the solvent is evaporated and the mixture is dried to obtain the microcapsules; (2) the grinding method, in which curcumin is added to a grinding solution of cyclodextrin and further ground, and the curcumin displaces the water in the cavity of the cyclodextrin, and then the mixture is dried to obtain the microcapsules; (3) the coprecipitation method, in which a cyclodextrin aqueous solution is mixed with a curcumin-organic reagent solution, the temperature of the liquid is increased and the liquid is stirred vigorously to saturate the mixture, then lower the temperature of the liquid to cause the cyclodextrin-curcumin complexes to crystallize and precipitate.
The precipitate is filtered, collected, and dried to obtain curcumin microcapsules. CN106943604A uses water as a solvent to mix cyclodextrin polymers with curcumin, and then spin-vacuum dries to obtain curcumin microcapsules with good water solubility [84]. Purpura et al. [85] used γ-cyclodextrin to encapsulate curcuminoids and studied the concentrations of the three curcuminoids in the blood plasma after oral administration before and after encapsulation. The results showed that γ-cyclodextrin encapsulation can significantly increase the concentration of curcuminoids in the blood plasma, i.e., improve the body's absorption of curcuminoids. Zhang et al. [86] used β-cyclodextrin as a wall material and prepared curcumin-cyclodextrin complexes by a saturated aqueous solution method. Compared with free curcumin, curcumin in the complex is more easily taken up by cells and has a better therapeutic effect on lung cancer.
2.1.4 Other preparation methods
In addition to the three commonly used preparation methods mentioned above, liposome encapsulation-electrospaying, the sharp pore method, and the isoelectric precipitation method are also used to prepare curcumin microcapsules. Liu Xin et al. [87] used chitosan as the wall material and prepared curcumin microcapsules by the sharp pore method. The resulting microcapsules were uniform in size, with an encapsulation rate of 60% and a drug loading of 0.75%. However, the particle size was large, and concentrated around 0.45 mm. The sharp-pore method is slow and unsuitable for industrial production, and there has been little research on its use in preparing curcumin microcapsules. Ariyarathna et al. [88] used chickpea protein as the wall material to prepare curcumin microcapsules based on the principle of isoelectric precipitation, with an encapsulation rate of 78.6% and a loading capacity of 9.2%. This significantly improved the light and thermal stability of curcumin, However, the method has limited choices of wall materials. Laura et al. [89] used electrospraying to encapsulate curcumin liposomes in whey protein. Free curcumin was almost completely degraded in phosphate buffer at pH 7.4 within 1 h. Under the same conditions, the curcumin retention rate of curcumin liposomes was about 80% at 25 h, while the retention rate of the electro-sprayed microcapsules was about 90% at 25 h, which shows that double-encapsulation gives turmeric curcuminoids stronger protection.
2.2 Main wall materials for turmeric curcuminoids microencapsulation
The wall material is the most important component of the microcapsule, apart from the core material, and it affects the physical and chemical properties of the microcapsule to a certain extent, such as the apparent shape, moisture content, product yield, solubility, permeability and sustained-release effect. Therefore, it is particularly important to choose the appropriate wall material according to the different core materials and preparation methods.
2.2.1 Proteins
Proteins are a type of natural macromolecular polymer in food, with excellent emulsifying and gelling properties. At present, common protein-based wall materials used in the preparation of curcumin microcapsules include soy protein isolate, whey protein, and zein.

2.2.1.1 Whey protein
Whey protein is the main component of whey, which is obtained by concentrating and refining whey, a by-product of cheese production. The main components are β-lactoglobulin, α-lactalbumin, immunoglobulins, and bovine serum albumin. Whey protein (WP) is mainly divided into two categories: whey protein concentrate (WPC) and whey protein isolate (WPI). It has excellent film-forming, emulsifying and gelling properties, and is often used as a carrier material for bioactive substances. Jayaprakasha et al. [73] used whey protein as the wall material and prepared turmeric curcumin microcapsules by freeze drying, with an encapsulation rate of 96.34%. After nano-encapsulation, WP-Cur can maintain a micelle structure under neutral conditions, with a turmeric curcumin release of 599.49% at 24 hours and greater than 70% at 48 hours.
The uptake of curcumin by cells increased, and the anti-cancer activity against cancer cells (colon cancer cells SW480, prostate cancer cells LNCap) increased, i.e. the nano-encapsulation of whey protein reduced the metabolism of curcumin by delayed release and increased the bioavailability of curcumin. Whey protein can self-assemble at acidic pH (pH 2) and low ionic strength, and heat for several hours above the denaturation temperature to form fibrous aggregates with a diameter of about 1 to 10 nm and a length of microns. Compared with non-fibrous whey protein, whey protein nanofibrils (WPN) WPN) have higher radical scavenging activity and better emulsifying properties at low concentrations than non-fibrillar whey protein. WPN have higher surface hydrophobicity and are more likely to form soluble complexes with curcumin through hydrogen bonding and hydrophobic interactions. Compared with free curcumin, the solubility of curcumin in WPI-Cur is increased by about 180 times, and the solubility of curcumin in WPN-Cur is increased by about 1200 times. In addition, the combination of curcumin and WPN further increases the apparent viscosity and surface activity of WPN. Therefore, Cur-WPN may be an ideal choice for designing new functional food emulsions and beverages [90]. Hu et al. also had similar research results [91].
2.2.1.2 Zea mays alcohol-soluble protein
Zein is composed of about 75% hydrophobic amino acid residues and 25% hydrophilic amino acid residues. It is amphiphilic and a kind of plant protein that is soluble in alcohol and insoluble in water. Under external induction, zein can self-assemble into nanoparticles, encapsulating hydrophobic active substances inside to form a core-shell structure. However, microcapsules prepared from Zein alone are prone to aggregation and bursting, leading to the release of the active substance. Therefore, Zein is usually combined with polysaccharide wall materials as a carrier material. For example, Li [80] used Zein and Chitosan (CS) as wall materials to deliver curcumin. It was found that the higher the pH of the system, the stronger the interaction between Zein and CS, the higher the product yield, and the lower the in vitro release rate of curcumin. Considering all factors, Zein-CS-Cur has better oral administration potential at pH = 4. In Ran et al. [92], the half-life t1/2 value of curcumin under light irradiation conditions in Zein-hydroxypropyl methylcellulose-Cur was increased, and the DPPH radical scavenging rate increased from 19.56% to 68.25%. In recent years, Maillard reaction products have also been widely used to encapsulate bioactive substances. Dong Xiao et al. [93] used Zein and glucose (Glu) in a 70% ethanol solution of the Maillard reaction product (Zein/Glu MRP) to prepare curcumin nanocapsules. Compared to Zein, the Zein/Glu MRP prepared curcumin nanocapsules had a 22-fold increase in entrapment efficiency, and the thermal stability and storage stability were significantly improved.
2.2.1.3 Other protein-based wall materials
In addition to the above-mentioned proteins, soy protein isolate, coconut protein isolate, pea protein, egg white protein, gelatin, etc. have also been used to prepare curcumin microcapsules. For example, Chen et al. [94] used soy protein isolate (SPI) as a wall material to prepare curcumin microcapsules by spray drying. After spray drying, the retention rate of curcumin was 89.1%, and the loading capacity was 25.3 mg/g. Scanning electron microscopy images showed that the surface of the microcapsules had large, regular indentations. After adding soy polysaccharides and/or maltodextrin to the wall material, the retention rate, loading and solubility of curcumin were significantly improved. The microcapsule membrane protected curcumin to reduce degradation during spray drying, and SEM images showed that the surface of the microcapsules had fewer dents and folds and was smoother. Adsare et al. [95] used coconut clear protein to encapsulate curcumin, and the encapsulation rate of the spray-dried microcapsules was (84.89±1.09)%. the curcumin loading was 509.26 mg/100 g. When 5%, 10%, and 15% gum arabic was added to the wall material, the encapsulation rate and loading gradually increased. This may be due to the fact that gum arabic occupies the voids in the coconut clear protein wall material matrix, reducing oxygen permeability.
2.2.2 Carbohydrates
2.2.2.1 Gum arabic
Gum arabic (GA) is a mixture of polysaccharides and glycoproteins. It is non-toxic, highly soluble, has surface activity, is stable over a wide pH range and has relatively low viscosity. It is widely used in the food, cosmetics and pharmaceutical industries. Andreea et al. [96] used three different concentrations (10%, 15% and 20% w/v) of GA to encapsulate curcumin. the diameter of the microcapsules was 7–9 μm. With an increase in the proportion of GA, the release of curcumin from the microcapsules in simulated gastrointestinal fluid decreased and the release rate slowed down during the first few minutes. Within a certain range, increasing the shell-to-core ratio will increase the encapsulation rate and loading capacity of the microcapsules. However, a high concentration of GA will cause the feed liquid to become viscous, which is not conducive to spray drying [70]. can be improved by compounding GA with other wall materials, which will not have a significant effect on the viscosity of the feed liquid while improving the encapsulation efficiency [97-98]. For example, Meena et al. [66] combined GA with maltodextrin and WPC-80 to encapsulate curcumin, and the microcapsule encapsulation rate was up to 97.16%, and the curcumin content was 422.28 mg/kg. After simulated gastric digestion, about 88% of the curcumin was retained in the microcapsules. Tan Shaocong et al. [99] used GA and zein as wall materials to prepare curcumin microcapsules by freeze-drying, and the microcapsule entrapment rate was 95.844%, with a loading capacity of 62 mg/g.

2.2.2.2 Dextrins
Dextrin is a small molecule intermediate material that is converted from starch macromolecules through decomposition and hydrolysis under the action of heat, acid or enzymes. Among them, maltodextrin, cyclodextrin and their derivatives are commonly used as wall materials for microcapsules.
Maltodextrin (MD) is a sugar polymer prepared from starch or amylum as raw material by enzymatic low-degree hydrolysis, purification, and drying or non-drying [100]. The degree of hydrolysis is generally expressed by the DE value (glucose equivalent). The DE value is the percentage of directly reducing sugars (expressed as glucose) in the total solids of the starch hydrolysate. MDs with different DE values have different molecular weight distributions, average chain lengths, and degrees of branching, resulting in different functional properties, such as viscosity and hygroscopicity. The appropriate DE value of MD should be selected based on the characteristics of the core material and the preparation method. MD has the characteristics of low viscosity, low hygroscopicity, high solubility and low cost when used in high concentrations [101-102]. In the study of microencapsulation of curcumin, due to the poor emulsifying ability and emulsion stability of MD, the encapsulation rate and loading amount of microcapsules prepared with MD alone as the wall material are low [103-104]. Therefore, MD is usually compounded with wall materials with excellent emulsifying properties (see Table 2), such as gum arabic, whey protein, gelatin, etc.
Cyclodextrin (CD) is a series of cyclic oligosaccharides produced by the action of cyclodextrin glucanotransferase on straight-chain starch. It has a conical cavity with a ring shape (see Figure 5) [85]. The shielding effect of the primary hydroxyl groups on the outer surface of the cavity and the C-H bonds inside the cavity results in a structure with the characteristics of “hydrophobic inside cavity and hydrophilic outer wall”. Therefore, it can be used to embed some object molecules of appropriate size and shape in the cyclic structure through electrostatic interactions, van der Waals forces, hydrophobic interactions, hydrogen bonding, etc., to form microcapsules [108]. Cyclodextrins are non-toxic, inexpensive and widely available, making them ideal for use as carriers for active ingredients. Cyclodextrins commonly used to encapsulate curcumin are β-CD and γ-CD. Related research is shown in Table 3. Compared to pure curcumin, cyclodextrin encapsulation significantly improves curcumin solubility, stability and antioxidant activity (possibly due to the improved solubility of curcumin, which in turn increases the concentration of curcumin in the system). However, the microencapsulation rate is relatively low, and the solubility of β-CD in water is poor, at 1.85 mg/mL [109], which is not conducive to its good application in the field of carrier materials. At present, some studies have introduced chemical groups to cyclodextrins to obtain modified cyclodextrins, thereby improving their solubility and encapsulation properties. After modification, the encapsulation rate of curcumin by cyclodextrin was significantly improved, and the stability of the Cur-CD complex was also improved, and the dissolution and stability of curcumin in the complex were further improved [108, 110-111].
2.2.2.3 Modified starch
Starch is one of the most abundant carbohydrates in nature and is also the main nutrient that supplies energy to the human body. It is a safe, non-toxic, biocompatible, low-cost and abundant source of nutrients. Natural starch has poor solubility, and using it directly as a microcapsule wall material is not very effective. The encapsulation rate and loading capacity of microcapsules are relatively low. Therefore, in the application of microcapsule wall materials, the natural properties of starch are often modified through physical, chemical or enzymatic treatments to increase certain functions or introduce new properties, so as to improve its solubility, water absorption and encapsulation capacity, and make it a good microcapsule wall material. Table 4 shows the relevant research on the preparation of curcumin microcapsules using modified starch as the wall material. Compared with natural starch, modified starch can significantly improve the encapsulation rate and loading capacity of the microcapsules, as well as the solubility, stability and bioavailability of curcumin, improving the controlled release effect of curcumin from the microcapsules. Although the microcapsules prepared with modified starch are of better quality than those prepared with natural starch, there have been relatively few studies on whether modified starch-curcumin microcapsules have adverse effects on human health [115-121]. However, in the delivery system of active substances, the toxicity of the carrier matrix is an important issue that requires further research.
3 Applications
GB 2760—2014 [122] stipulates that curcumin, as a natural edible pigment, can be used in frozen drinks, cooked nuts and seeds, chocolate products, candy, instant rice and noodle products, fillings for grain products, flavored syrups, compound seasonings, carbonated drinks, jelly and puffed foods. At present, some scholars have also added curcumin microcapsules to yogurt, cheese and milk and evaluated their suitability. Patel et al. [123] prepared curcumin microcapsules using WPI and Hi-Cap 100 as wall materials, and added WPI, Hi-Cap 100, a physical mixture of curcumin and microcapsules to milk, respectively. the milk with the physical mixture had obvious particle sedimentation and a lower sensory evaluation score, while the addition of curcumin microcapsules had no adverse effect on the sensory characteristics of the milk. Vanessa et al. [124] used β-CD to encapsulate curcumin and added β-CD-Cur to cheese (β-CD-Cur added at 5 × 10-7 g/L) and yogurt (β-CD-Cur added at 2 × 10-6 g/g) to evaluate its suitability.
The experimental results showed that the addition of curcumin complexes had no significant effect on the hardness, adhesion, elasticity, etc. of cheese and yogurt, but reduced the brightness of the two products, with the cheese turning yellow and the yogurt turning slightly yellowish-green. In addition, the sensory evaluation showed that the cheese with added β-CD-Cur was better accepted. Microencapsulation technology has broadened the scope of application of curcumin, making it suitable for use in some water-based foods. For example, functional dairy products or beverages. In addition, based on the superior bioactive function of curcumin, curcumin microcapsules can also be combined with other nutrients to make capsule or tablet-type functional supplements, or added to daily chemical products that focus on anti-inflammatory effects. It can be seen that curcumin and its microcapsules have broad development prospects in the fields of food health, medicine and daily chemical products.

4 Conclusion and outlook
Curcumin is a secondary metabolite of the ginger plant Curcuma longa. It is known as “liquid gold” and has a variety of biological activities, such as anti-oxidation, anti-inflammation, and anti-tumor. Therefore, there is broad potential for the development of curcumin-based functional foods. However, its unstable physicochemical properties, low solubility, and rapid metabolism limit its application. A technology is urgently needed to solve this problem. Therefore, this paper starts with the structural properties, biological activity, and metabolic characteristics of curcumin, and summarizes the common embedding techniques and types of wall materials used to prepare curcumin microcapsules in recent years.
A large number of studies have shown that microencapsulation technology can significantly improve the solubility of curcumin, enhance its stability to light, heat, oxygen and pH, and also have a sustained-release effect on curcumin in simulated gastrointestinal fluids. Among them, spray drying is a traditional method of microcapsule preparation that is relatively mature and is very suitable for the large-scale industrial production of curcumin microcapsules. There have been many studies on curcumin microcapsules, but there are still some problems in the current research: (1) Whether the microcapsules can still ensure the stability and bioavailability of curcumin in the food matrix and whether it will affect the original flavor of the food.
There is still very little research in this area and it needs to be studied in depth. (2) Although natural wall materials have the advantage of being biocompatible, their properties are unstable. Therefore, more modified wall materials have been applied in the preparation of curcumin microcapsules. However, in the research on curcumin microcapsules, it is rarely mentioned whether modified wall materials have adverse effects on human health. Therefore, research on the toxicity of the carrier matrix needs to be improved. (3) The loading capacity of curcumin microcapsules is low, and the industrial production technology for high-loading curcumin microcapsules is not yet mature in China. With the innovation of microencapsulation technology, it is believed that these problems can be solved in the future.
Reference:
[1]Guo S P ,Chang H C ,Lu L S ,et al. Activation of kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2/antioxidant response element pathway by curcumin enhances the anti-oxidative capacity of corneal endothelial cells [J]. Biomedicine & Pharmacotherapy = Biomedecine &Pharmacotherapie ,2021 ,141 :111834.
[2]Rai A ,Kaur M ,Gombra V ,et al. Comparative evaluation of curcumin and antioxidants in the management of oral submucous fibrosis [J]. Journal of Investigative and Clinical Dentistry, 2019 ,10(4):e12464.
[3] Zheng Q T ,Yang Z H ,Yu L Y ,et al. Synthesis and antioxidant activity of curcumin analogs [J]. Journal of Asian Natural Products Research ,2017 ,19(5):489-503.
[4]Momeni H R ,Eskandari N. Effect of curcumin on kidney histopathological changes ,lipid peroxidation and total antioxidant capacity of serum in sodium arsenite-treated mice [J]. Experimental and Toxicologic Pathology ,2017 ,69(2): 93-97.
[5]Wang Y,Wang Y J ,Cai N ,et al. Anti-inflammatory effects of curcumin in acute lung injury :In vivo and in vitro experimental model studies [J]. International Immunopharmacology ,2021, 96 :107600.
[6]Wang Q R ,Ye C Q ,Sun S K ,et al. Curcumin attenuates collagen-induced rat arthritis via anti-inflammatory and apoptotic effects [J] . International Immunopharmacology, 2019 ,72:292-300.
[7] Shimizu K ,Funamoto M ,Sunagawa Y ,et al. Anti inflammatory action of curcumin and its use in the treatment of lifestyle-related diseases [J]. European Cardiology,2019 ,14 (2):117-122.
[8]Ebrahimzadeh A ,Abbasi F ,Ebrahimzadeh A ,et al. Effects of curcumin supplementation on inflammatory biomarkers in patients with Rheumatoid Arthritis and Ulcerative colitis :A systematic review and meta-analysis [J] . Complementary Therapies in Medicine ,2021 ,61 :102773.
[9]Nguyen-Ngo C,Willcox J C ,Lappas M. Anti-inflammatory effects of phenolic acids punicalagin and curcumin in human placenta and adipose tissue [J]. Placenta ,2020 ,100 :1-12.
[10] Li Y P ,Sun W X ,Han N ,et al. Curcumin inhibits proliferation,migration ,invasion and promotes apoptosis of retinoblastoma cell lines through modulation of miR-99a and JAK/STAT pathway [J]. BMC Cancer ,2018 ,18(1):1230.
[11]Wang Z X ,Liu F ,Liao W L ,et al. Curcumin suppresses glioblastoma cell proliferation by p-AKT/mTOR pathway and increases the PTEN expression [J]. Archives of Biochemistry and Biophysics ,2020 ,689(prepublish):108412.
[12]Fan X ,Zhu M ,Qiu F ,et al. Curcumin may be a potential adjuvant treatment drug for colon cancer by targeting CD44 [J]. International Immunopharmacology ,2020 ,88 :106991.
[13]Elbadawy M ,Hayashi K ,Ayame H ,et al. Anti-cancer activity of amorphous curcumin preparation in patient- derived colorectal cancer organoids [J] . Biomedicine & Pharmacotherapy ,2021 ,142 :112043.
[14]Liu Q ,Loo W TY ,Sze S C W ,et al. Curcumin inhibits cell proliferation of MDA-MB-231 and BT-483 breast cancer cells mediated by down-regulation of NFkappaB ,cyclinD and MMP-1 transcription [J]. Phytomedicine :International Journal of Phytotherapy and Phytopharmacology ,2009 ,16 (10):916-922.
[15]Zhang P L ,Zuo Z G ,Wu A H ,et al. miR-600 inhibits cell proliferation,migration and invasion by targeting p53 in mutant p53-expressing human colorectal cancer cell lines [J]. Oncology Letters ,2017 ,13(3):1789-1796.
[16]Dubey R. Microencapsulation technology and applications [J]. Defence Science Journal ,2009 ,59 :82-95.
[17]Ghosh S,Banerjee S ,Sil P C. The beneficialrole of curcumin on inflammation ,diabetes and neurodegenerative disease :A recent update [J]. Food and Chemical Toxicology ,2015 ,83 : 111-124.
[18] Prasad S ,Gupta S C ,Tyagi A K ,et al. Curcumin ,a component of golden spice :From bedside to bench and back [J]. Biotechnology Advances ,2014 ,32(6):1053-1064.
[19] Priyadarsini K I . Photophysics ,photochemistry and photobiology of curcumin :Studies from organic solutions, bio-mimetics and living cells [J]. Journal of Photochemistry and Photobiology C :Photochemistry Reviews ,2009 ,10 (2):81-95.
[20] Tønne sen H H ,Karl sen J . Studies on curcumin and curcuminoids [J]. Zeitschrift Für Lebensmittel-Untersuchung Und Forschung ,1985 ,180(5):402-404.
[21]Zhang L ,Zhu W W ,Yang C F ,et al. A novel folate- modified self-microemulsifying drug delivery system of curcumin for colon targeting [J] . International Journal of Nanomedicine ,2012 ,7 :151-162.
[22]Vuki evi M ,Tønnesen H H. Interaction between curcumin and human serum albumin in the presence of excipients and the effect of binding on curcumin photostability [J] . Pharmaceutical Development and Technology ,2016 ,21 (4):428-436.
[23] Suvarna S ,Dsouza J ,Ragavan M L ,et al. Potential probiotic characterization and effect of encapsulation of probiotic yeast strains on survival in simulated gastrointestinal tract condition [J]. Food Science and Biotechnology ,2018, 27(3):745-753.
[24]Tønnesen H H,Karlsen J,van Henegouwen G B. Studies on curcumin and curcuminoids VIII. photochemical stability of curcumin [J]. Zeitschrift Für Lebensmittel-Untersuchung Und Forschung ,1986 ,183(2):116-122.
[25]del Castillo M L R ,López-Tobar E ,Sanchez-Cortes S, et al. Stabilization of curcumin against photodegradation by encapsulation in gamma-cyclodextrin :A study based on chromatographic and spectroscopic(Raman and UV–visible) data [J]. Vibrational Spectroscopy ,2015 ,81 :106-111.
[26]Wang Y J,Pan M H ,Cheng A L ,et al. Stability of curcumin in buffer solutions and characterization of its degradation products [J] . Journal of Pharmaceutical and Biomedical Analysis ,1997 ,15(12):1867-1876.
[27]Tønnesen H H ,Másson M ,Loftsson T. Studies of curcumin and curcuminoids . XXVII. Cyclodextrin complexation : Solubility ,chemical and photochemical stability [J] . International Journal of Pharmaceutics ,2002 ,244(1-2): 127-135.
[28]Martínez-Guerra J. New insights on the chemical stability of curcumin in aqueous media at different pH :Influence of the experimental conditions [J]. International Journal of Electrochemical Science ,2019 :5373-5385.
[29] Dinkova-Kostova A T ,Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins [J]. Molecular Nutrition & Food Research ,2008 ,52(Suppl 1):S128-S138.
[30] Barclay L R ,Vinqvist M R ,Mukai K ,et al. On the antioxidant mechanism of curcumin :Classical methods are needed to determine antioxidant mechanism and activity [J]. Organic Letters ,2000 ,2(18):2841-2843.
[31] Sun Y M ,Zhang H Y ,Chen D Z ,et al. Theoretical elucidation on the antioxidant mechanism of curcumin :A DFT study [J]. Organic Letters ,2002,4(17):2909-2911.
[32]Priyadarsini K I ,Maity D K ,Naik G H ,et al. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin [J]. Free Radical Biology and Medicine ,2003 ,35(5):475-484.
[33]Jovanovic S V ,Steenken S ,Boone C W ,et al. H-atom transfer is a preferred antioxidant mechanism of curcumin [J]. Journal of the American Chemical Society ,1999 ,121(41): 9677-9681.
[34]Deng S L ,Chen W F ,Zhou B ,et al. Protective effects of curcumin and its analogues against free radical-induced oxidative haemolysis of human red blood cells [J] . Food Chemistry ,2006 ,98(1):112-119.
[35] Sun Yiyang, Peng Ziyi, Zhao Yuxin, et al. Research progress on the anti-inflammatory effect of curcumin in disease treatment [J]. China Medical Innovation, 2021, 18(27): 181-184.
[36] Zhang B, Ye L. Research progress on the anti-cancer mechanism of curcumin [J]. Journal of Traditional Chinese Medicine, 2013, 41(1): 121-123.
[37] Ren J Y, Gou N, Gao L, et al. Effect of curcumin on Helicobacter pylori and its damage to human gastric GES-1 cells [J]. Food Science, 2019, 40 (23): 151-156.
[38] Xu Chuanjun, Ming Yanlin, Chen Lianghua, et al. Research progress on the role and mechanism of curcumin in type 2 diabetes and its chronic complications [J]. Anhui Agricultural Science, 2021, 49 (6): 30-34, 38.
[39]Kuroda M ,Mimaki Y,Nishiyama T ,et al. Hypoglycemic effects of turmeric(Curcuma longa L. rhizomes)on genetically diabetic KK-Ay mice [J] . Biological & Pharmaceutical Bulletin ,2005 ,28(5):937-939.
[40] Holder G M ,Plummer J L ,Ryan A J. The metabolism and excretion of curcumin(1 ,7-bis-(4-hydroxy-3 - methoxyphenyl)-1,6-heptadiene-3,5-dione)in the rat [J]. Xenobiotica ,1978 ,8(12):761-768.
[41] Pandey A ,Chaturvedi M ,Mishra S ,et al. Reductive metabolites of curcumin and their therapeutic effects [J] . Heliyon ,2020 ,6(11):e05469.
[42]Liu W D,Zhai Y J,Heng X Y ,et al. Oral bioavailability of curcumin :Problems and advancements [J]. Journal of Drug Targeting ,2016 ,24(8):694-702.
[43]Wang K ,Qiu F. Curcuminoid metabolism and its contribution to the pharmacological effects [J]. Current Drug Metabolism, 2013 ,14(7):791-806.
[44] Shi M G ,Gao T T ,Zhang T ,et al. Characterization of curcumin metabolites in rats by ultra-high-performance liquid chromatography with electrospray ionization quadrupole time-of-flight tandem mass spectrometry [J] . Rapid Communications in Mass Spectrometry :RCM ,2019 ,33 (13):1114-1121.
[45]Vareed S K,Kakarala M,Ruffin M T ,et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects [J] . Cancer Epidemiology ,Biomarkers & Prevention :A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology ,2008 ,17(6):1411-1417.
[46] He Yongjian, Huang Shaowen, Liu Ruijing, et al. Intervention effect of medlar juice on the metabolism of di(2-ethylhexyl) phthalate based on the uridine diphosphate glucuronic acid transferase 1 detoxification pathway [J]. Food Science, 2017, 38(23): 196-200.
[47] Hoehle S I ,Pfeiffer E ,Metzler M. Glucuronidation of curcuminoids by human microsomaland recombinant UDP- glucuronosyltransferases [J] . Molecular Nutrition & Food Research ,2007 ,51(8):932-938.
[48] Strassburg C P ,Nguyen N ,Manns M P ,et al. UDP- glucuronosyltransferase activity in human liver and colon [J]. Gastroenterology ,1999 ,116(1):149-160.
[49 ] Tu key R H , St ra ssbu r g C P . Hum a n UDP - glucuronosyltransferases :Metabolism ,expression ,and disease [J]. Annual Review of Pharmacology and Toxicology, 2000,40 :581-616.
[50]Mueller J W ,Idkowiak J ,Gesteira T F ,et al. Human DHEA sulfation requires direct interaction between PAPS synthase 2 and DHEA sulfotransferase SULT2A1 [J] . Journal of Biological Chemistry ,2018 ,293(25):9724-9735.
[51] Lu X Y ,Jiang K Y ,Han L ,et al. Sulfonation of curcuminoids :Characterization and contribution of individual SULT enzymes [J]. Molecular Nutrition & Food Research, 2015 ,59(4):634-645.
[52]Burapan S ,Kim M ,Han J. Curcuminoid demethylation as an alternative metabolism by human intestinal microbiota [J]. Journal of Agricultural and Food Chemistry ,2017 ,65(16): 3305-3310.
[53] Tan S ,Calani L ,Bresciani L ,et al. The degradation of curcuminoids in a human faecal fermentation model [J] . International Journal of Food Sciences and Nutrition ,2015, 66(7):790-796.
[54]Niwa T ,Yokoyama S I ,Mochizuki M ,et al. Curcumin metabolism by human intestinal bacteria in vitro [J]. Journal of Functional Foods ,2019 ,61 :103463.
[55]An C Y ,Sun Z Z ,Shen L ,et al. Biotransformation of food spice curcumin by gut bacterium Bacillus megaterium DCMB- 002 and its pharmacological implications [J]. Food & Nutrition Research ,2017 ,61(1):1412814.
[56]Zam W. Gut microbiota as a prospective therapeutic target for curcumin :A review of mutual influence [J]. Journal of Nutrition and Metabolism ,2018 ,2018 :1367984.
[57]Di Meo F,Margarucci S ,Galderisi U ,et al. Curcumin ,gut microbiota ,and neuroprotection [J]. Nutrients ,2019 ,11(10):2426.
[58]Pluta R ,Januszewski S ,U amek-Kozio M. Mutual two-way interactions of curcumin and gut microbiota [J] . International Journal of Molecular Sciences ,2020 ,21(3): 1055.
[59] Scazzocchio B ,Minghetti L ,D’Archivio M. Interaction between gut microbiota and curcumin :A new key of understanding for the health effects of curcumin [J]. Nutrients, 2020 ,12(9):2499.
[60]Wahlström B,Blennow G. A study on the fate of curcumin in the rat [J]. Acta Pharmacologica et Toxicologica ,1978 ,43(2):86-92.
[61] Pan M H ,Huang T M ,Lin J K. Biotransformation of curcumin through reduction and glucuronidation in mice [J]. Drug Metabolism and Disposition :the Biological Fate of Chemicals ,1999 ,27(4):486-494.
[62]Corrêa Filho L C ,Martins M M ,Alves V D. Advances in the application of microcapsules as carriers of functional compounds for food products [J]. Applied Sciences ,2019 ,9 (3):571.
[63] Adachi S ,Imaoka H ,Ashida H ,et al. Preparation of microcapsules of W/O/W emulsions containing a polysaccharide in the outer aqueous phase by spray-drying [J]. European Journal of Lipid Science and Technology ,2004, 106(4):225-231.
[64] Hu Zhihe, Zhao Yong, Xia Lei, et al. Effect of different drying methods on the activity of the angiotensin-converting enzyme inhibitory peptide from milk [J]. Food Science, 2016, 37 (19): 204-210.
[65] Wu Kegang, Chai Xianghua. Study on the embedding performance of single-cell AA oil spray-dried microcapsule wall materials [J]. Journal of Chemical Engineering of Universities, 2008 (5): 797-802.
[66] Meena S ,Prasad W ,Khamrui K ,et al. Preparation of spray-dried curcumin microcapsules using a blend of whey protein with maltodextrin and gum Arabica and its in-vitro digestibility evaluation [J] . Food Bioscience ,2021 ,41 : 100990.
[67]Zuanon LAC,Malacrida C R,Telis VRN. Effect of ultrasound on the stability of turmeric oleoresin microencapsulated in gelatin-collagen matrices [J] . Journal of Food Process Engineering,2017,40(2):e12360.
[68] Zheng Ju, Zhao Lei, Wang Kai, et al. Effect of spray drying conditions on the survival rate of Lactobacillus in lychee milk powder and the characteristics of the powder [J]. Food Industry Science and Technology, 2017, 38 (9): 216-220, 227.
[69] Taki M ,Tagami T ,Fukushige K ,et al. Fabrication of nanocomposite particles using a two-solution mixing-type spray nozzle for use in an inhaled curcumin formulation [J]. International Journal of Pharmaceutics ,2016 ,511(1): 104-110.
[70]Nguyen V T,Huynh T M,Nguyen T N Q ,et al. Enhancing the stability of synthesized curcumin by spray-drying microencapsulation with soy lecithin and gum Arabic [J] . Brazilian Journal of Chemical Engineering ,2021 ,38(3): 563-572.
[71]Cano-Higuita D M ,Malacrida C R ,Telis V R N. Stability of curcumin microencapsulated by spray and freeze drying in binary and ternary matrices of maltodextrin ,gum Arabic and modified starch [J] . Journal of Food Processing and Preservation ,2015 ,39(6):2049-2060.
[72] de Andrade D F ,Vukosavljevic B ,Hoppe J B ,et al.Redispersible spray-dried powder containing nanoencapsulated curcumin :The drying process does not affect neuroprotection In vitro [J]. AAPS PharmSciTech,2019,20(7):283.
[73]Jayaprakasha G K ,Chidambara Murthy K N ,Patil B S. Enhanced colon cancer chemoprevention of curcumin by nanoencapsulation with whey protein [J]. European Journal of Pharmacology ,2016 ,789:291-300.
[74]Kayitmazer A B. Thermodynamics of complex coacervation [J]. Advances in Colloid and Interface Science,2017 ,239 : 169-177.
[75] Pathak J ,Priyadarshini E ,Rawat K ,et al. Complex coacervation in charge complementary biopolymers : Electrostatic versus surface patch binding [J]. Advances in Colloid and Interface Science ,2017 ,250:40-53.
[76]Mattison K W ,Dubin P L ,Brittain I J. Complex formation between bovine serum albumin and strong polyelectrolytes : effect of polymer charge density [J]. The Journal of Physical Chemistry B ,1998 ,102(19):3830-3836.
[77]Williams PA,Phillips G O. Introduction to food hydrocolloids [M]//Handbook of Hydrocolloids. Amsterdam :Elsevier, 2009 :1-22.
[78]Mohammadian M ,Salami M ,Alavi F ,et al. Fabrication and characterization of curcumin-loaded complex coacervates made of gum Arabic and whey protein nanofibrils [J]. Food Biophysics ,2019 ,14(4):425-436.
[79] Kavousi H R ,Fathi M ,Goli S A H. Novel cress seed mucilage and sodium caseinate microparticles for encapsulation of curcumin :An approach for controlled release [J]. Food and Bioproducts Processing ,2018 ,110 :126-135.
[80]Li M F ,Chen L ,Xu M Z ,et al. The formation of zein- chitosan complex coacervated particles :Relationship to encapsulation and controlled release properties [J] . International Journal of Biological Macromolecules ,2018, 116 :1232-1239.
[81] Shahgholi an N ,Rajabz a deh G . Fabrication and characterization of curcumin-loaded albumin/gum Arabic coacervate [J]. Food Hydrocolloids ,2016 ,59 :17-25.
[82]Xie H J,Xiang C Y ,Li Y ,et al. Fabrication of ovalbumin/ κ-carrageenan complex nanoparticles as a novel carrier for curcumin delivery [J]. Food Hydrocolloids ,2019 ,89 :111- 121.
[83] Chen Hongyan, Peng Zhongli, Xiao Dingshu. Adsorption behavior of β-cyclodextrin and cinnamaldehyde. Journal of Guangzhou University: Natural Science Edition, 2012, 11(4): 31-36.
[84] Chen Jianping, Peng Wanyi, Qin Xiaoming, et al. A method for preparing a curcumin cyclodextrin supramolecular complex: CN106943604A [P]. 2017.
[85]Purpura M ,Lowery R P ,Wilson J M ,et al. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects [J]. European Journal of Nutrition ,2018 ,57(3):929-938.
[86] Zhang L L ,Man S L ,Qiu H N ,et al. Curcumin - cyclodextrin complexes enhanced the anti-cancer effects of curcumin [J]. Environmental Toxicology and Pharmacology, 2016,48 :31-38.
[87] Liu Xin, Lang Kaikai, Qiu Juan, et al. Preparation of chitosan-curcumin microcapsules by the sharp pore method [J]. Journal of Tangshan Normal University, 2017, 39(2): 15-19.
[88] Ariyarathna I R ,Karunaratne D N. Microencapsulation stabilizes curcumin for efficient delivery in food applications [J]. Food Packaging and Shelf Life ,2016 ,10 :79-86.
[89]Gómez-Mascaraque L G ,Casagrande Sipoli C ,de La Torre L G ,et al. Microencapsulation structures based on protein- coated liposomes obtained through electrospraying for the stabilization and improved bioaccessibility of curcumin [J]. Food Chemistry ,2017 ,233 :343-350.
[90]Mohammadian M ,Salami M,Momen S M ,et al. Enhancing the aqueous solubility of curcumin at acidic condition through the complexation with whey protein nanofibrils [J] . Food Hydrocolloids ,2019 ,87 :902-914.
[91]Hu Y ,He C X ,Jiang C J ,et al. Complexation with whey protein fibrils and chitosan :A potential vehicle for curcumin with improved aqueous dispersion stability and enhanced antioxidant activity [J]. Food Hydrocolloids ,2020 ,104 : 105729.
[92] Meng R ,Wu Z Z ,Xie Q T ,et al. Preparation and characterization of zein/carboxymethyl dextrin nanoparticles to encapsulate curcumin :Physicochemical stability ,antioxidant activity and controlled release properties [J]. Food Chemistry, 2021 ,340 :127893.
[93] Dong Xiao, Huang Guoqing, Xiao Junxia. Preparation of curcumin nanoparticles by the Maillard reaction product of zein and glucose [J]. Chinese Journal of Food Science, 2021, 21 (3): 118-127.
[94] Chen F P ,Liu L L ,Tang C H . Spray -drying microencapsulation of curcumin nano complexes with soy protein isolate :Encapsulation ,water dispersion, bioaccessibility and bioactivities of curcumin [J] . Food Hydrocolloids ,2020 ,105 :105821.
[95]Adsare S R,Annapure U S. Microencapsulation of curcumin using coconut milk whey and Gum Arabic [J]. Journal of Food Engineering ,2021 ,298 :110502.
[96] Bucure s cu A ,Blaga A C ,Estevinho B N ,et al. Microencapsulation of curcumin by a spray-drying technique using gum Arabic as encapsulating agent and release studies [J] . Food and Bioprocess Technology ,2018 ,11(10): 1795-1806.
[97]Murrieta-Pazos I ,Gaiani C ,Galet L ,et al. Food Powders : Surface and form characterization revisited [J]. Journal of Food Engineering ,2012 ,112(1-2):1-21.
[98]Rafiee Z ,Nejatian M ,Daeihamed M ,et al. Application of different nanocarriers for encapsulation of curcumin [J]. Critical Reviews in Food Science and Nutrition ,2019 ,59 (21):3468-3497.
[99] Tan Shaocong, Qiu Menghui, Huang Dejin, et al. Preparation of curcumin nanoparticles based on the combined technology of stabilizer and microencapsulation [J]. Food Research and Development, 2021, 42 (15): 112-118.
[100] State Administration for Market Regulation, Standardization Administration of the People's Republic of China. Starch sugars—Quality requirements—Part 6: Maltodextrins: GB/T 20882.6—2021 [S]. Beijing: China Standards Press, 2021.
[101] Pereira K C ,Ferreira D C M ,Alvarenga G F ,et al. Microencapsulação e liberação controlada por difusão de ingredientes alimentícios produzidos através da secagem por atomização :Revisão [J] . Brazilian Journal of Food Technology ,2018 ,21.
[102]Akhavan Mahdavi S ,Jafari S M ,Assadpoor E ,et al. Microencapsulation optimization of natural anthocyanins with maltodextrin ,gum Arabic and gelatin [J]. International Journal of Biological Macromolecules ,2016 ,85 :379- 385.
[103]Aniesrani Delfiya D S ,Thangavel K,Natarajan N ,et al. Microencapsulation of turmeric oleoresin by spray drying and in vitro release studies of microcapsules [J]. Journal of Food Process Engineering ,2015 ,38(1):37-48.
[104]Kshirsagar A C ,Yenge V B ,Sarkar A ,et al. Efficacy of pullulan in emulsification of turmeric oleoresin and its subsequent microencapsulation [J]. Food Chemistry ,2009, 113(4):1139-1145.
[105] Patel S S ,Pushpadass H A ,Franklin M E E ,et al. Micro encapsulation of cur cumin by spray drying : Characterization and fortification of milk [J]. Journal of Food Science and Technology ,2022 ,59(4):1326-1340.
[106]Köprüalan Ö , İlter I,Akyıl S ,et al. Improving the stability of oily turmeric extract by microencapsulation using spray drying technique [J] . Journal of Dispersion Science and Technology ,2022,43(14):2240-2249.
[107]Ferreira S ,Malacrida C R ,Nicoletti V R. Influence of emulsification methods and spray drying parameters on the microencapsulation of turmeric oleoresin [J]. Emirates Journal of Food and Agriculture ,2019:491.
[108] Shityakov S ,Salmas R E ,Durdagi S ,et al. Solubility profiles,hydration and desolvation of curcumin complexed with γ-cyclodextrin and hydroxypropyl-γ-cyclodextrin [J]. Journal of Molecular Structure ,2017 ,1134 :91-98.
[109] Shinde V V ,Jeong D ,Jung S . Supramolecular aminocatalysis via inclusion complex :Amino-doped β-cyclodextrin as an efficient supramolecular catalyst for the synthesis of chromeno pyrimido [1 ,2-b]indazolin water [J]. Journal of Industrial and Engineering Chemistry ,2018, 68 :6-13.
[110]Hu Y ,Qiu C ,Julian McClements D ,et al. Encapsulation,protection ,and delivery of curcumin using succinylated- cyclodextrin systems with strong resistance to environmental and physiological stimuli [J]. Food Chemistry ,2022 ,376 : 131869.
[111] Liu C H ,Lee G W ,Wu W C ,et al. Encapsulating curcumin in ethylene diamine-β-cyclodextrin nanoparticle improves topical cornea delivery [J]. Colloids and Surfaces B, Biointerfaces ,2020 ,186 :110726.
[112] Sharma D ,Satapathy B K. Fabrication of optimally controlled electrosprayed polymer-free nano-particles of curcumin/β-cyclodextrin inclusion complex [J] . Colloids and Surfaces A :Physicochemical and Engineering Aspects, 2021 ,618 :126504.
[113] Lai D N ,Zhou A R ,Tan B K ,et al. Preparation and photodynamic bactericidal effects of curcumin-β-cyclodextrin complex [J]. Food Chemistry,2021 ,361 :130117.
[114] Arya P ,Raghav N. In-vitro studies of Curcumin-β - cyclodextrin inclusion complex as sustained release system [J]. Journal of Molecular Structure ,2021 ,1228 :129774.
[115]Athira G K ,Jyothi A N,Vishnu V R. Water soluble octenyl succinylated cassava starch-curcumin nanoformulation with enhanced bioavailability and anticancer potential [J]. Starch - Stärke ,2018 ,70(7-8):1700178.
[116] Miskeen S ,An Y S ,Kim J Y. Application of starch nanoparticles as host materials for encapsulation of curcumin : Effect of citric acid modification [J]. International Journal of Biological Macromolecules ,2021 ,183 :1-11.
[117] Acevedo-Guevara L ,Nieto-Suaza L ,Sanchez L T, et al. Development of native and modified banana starch nanoparticles as vehicles for curcumin [J] . International Journal of Biological Macromolecules ,2018 ,111 :498- 504.
[118] Sanchez L T ,Arbelaez L M ,Villa C C. Comparison of the release kinetics of bioactive molecules from native and modified starch nanoparticles into food and gastric simulants [J]. Starch - Stärke ,2021 ,73(11-12):2100064.
[119]Li J L ,Shin G H ,Lee I W ,et al. Soluble starch formulated nanocomposite increases water solubility and stability of curcumin [J]. Food Hydrocolloids ,2016 ,56:41-49.
[120] My D T T ,Lan P N T ,Van H P. Comparison in morphology ,structure and functionality of curcumin-loaded starch nanoparticles fabricated from short,medium and long chain-length debranched cassava starches [J]. International Journal of Food Science & Technology ,2021 ,57(11): 6913-6924.
[121]Park H R ,Rho S J ,Kim Y R. Solubility ,stability ,and bioaccessibility improvement of curcumin encapsulated using 4-α-glucanotransferase-modified rice starch with reversible pH-induced aggregation property [J]. Food Hydrocolloids, 2019 ,95 :19-32.
[122] National Health and Family Planning Commission of the People's Republic of China. National Food Safety Standard: Food Additive Use Standard: GB 2760-2014 [S]. Beijing: China Standard Press, 2015.
[123] Patel S S ,Pushpadass H A ,Franklin M E E ,et al. Micro encapsulation of cur cumin by spray drying : Characterization and fortification of milk [J]. Journal of Food Science and Technology ,2022 ,59(4):1326-1340.
[124]Marcolino VA,Zanin G M,Durrant L R ,et al. Interaction of curcumin and bixin with β-cyclodextrin :Complexation methods ,stability ,and applications in food [J]. Journal of Agricultural and Food Chemistry ,2011 ,59(7):3348- 3357.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese