Study on the Natural Food Coloring Curcumin Bioavailability
The use of natural dyes in China can be traced back more than 4,500 years ago, and has a very long history. At the end of the 20th century, chemical dyes gradually replaced natural dyes. Synthetic dyes are widely used in the textile, food, and industrial industries because of their many advantages, such as bright colors, good coloring power, good stability, low price, and large production. However, synthetic dyes can harm the environment and humans during production and use, and are currently one of the main pollutants in industrial wastewater [1]. Many synthetic dyes are extremely harmful to humans and living organisms during use, such as causing allergies, toxicity, mutagenicity and carcinogenicity [2-3]. Natural dyes come from plants and animals, and have the advantages of being safe and environmentally friendly, harmless to humans and living organisms, and having various health benefits. Therefore, they are favored by people and are gradually replacing synthetic pigments [4-6].
However, natural pigments also have problems such as unstable storage and transportation, easy hydrolysis, and low dyeing efficiency [7]. At present, the methods used to solve these problems include microencapsulation technology, the addition of antioxidants, the addition of pigment stabilizers, and the chemical modification of the structural groups of natural pigments. However, there have been few studies on using metal-organic framework materials (MOFs) to solve these problems [8-10]. Therefore, how to use new methods to solve these problems has become the research direction today.

This paper systematically lists the structure and properties of the natural pigment curcumin, and provides a detailed introduction to its applications. It focuses on summarizing the specific factors that affect the stability of curcumin and methods for improving it. A new method is introduced to encapsulate curcumin to improve its stability and dyeing properties. In order to provide more possibilities for solving the problems of unstable storage and transportation, easy hydrolysis, and low dyeing efficiency of curcumin, the future research prospects of curcumin in the textile field are proposed.
1 Curcumin and its properties
1.1 Molecular structure
Curcumin is a rare orange-yellow crystalline natural substance of the diketone class extracted from plants in the ginger family. Curcumin is its main substance, and its composition mainly includes about 77% curcumin (C12H20O6), 3% bisdemethoxycurcumin, and 17% demethoxycurcumin (C20H18O5) [11], with the following structure.
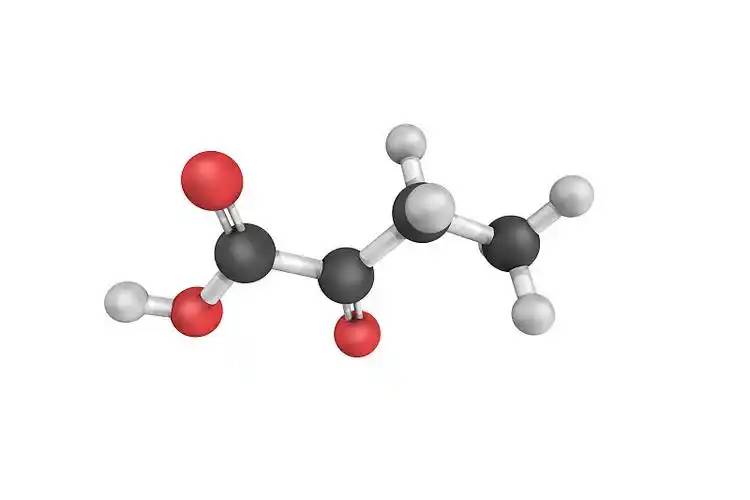
1.2 General properties
Turmeric extract is a natural compound with a characteristic aroma and a slightly bitter taste. It has a melting point of 179–182 °C, is highly lipophilic, and is very poorly soluble in water. It is readily soluble in methanol, ethanol, propanol, glacial acetic acid, and alkaline solutions. The pH has a significant effect on the color development of curcumin. It is yellow in neutral and acidic solutions and red in alkaline solutions. Given the color change of curcumin in acid and alkaline solutions, it can be used as an indicator of acidity and alkalinity. Because there are multiple double bonds, phenolic hydroxyl groups and carbonyl groups in the curcumin molecule, it undergoes relatively strong chemical reactions [12]. Therefore, curcumin is susceptible to the influence of physicochemical factors such as light and heat in applications.
1.3 Factors affecting stability
Turmeric pigment stability is easily affected by light, heat, pH and metal ions, etc., and has the characteristics of low water solubility, decomposition during use, and poor light stability, which limits the application and development of turmeric pigment. Promoting the use of turmeric pigment must solve the stability problem.
1.3.1 Light and heat
Turmeric extract has poor stability to light and heat, which can promote its oxidative decomposition [13]. The stability of a solution of curcuminoids and compounds is relatively good under indoor lighting conditions and in a light box, and degradation is slow. However, after 5 h of light exposure
the absorbance decreased from 1.15 to 0.38, and the pigment loss was about 67% [14]. Shi Wenting et al. [15] found that after 5 days of exposure to open air light, the degradation rates of the main components of curcumin, curcumin, monodemethoxycurcumin, and bisdemethoxycurcumin, were 69.5%, 51.4%, and 21.2%, respectively.
Turmeric pigments degrade slowly at low temperatures, but the rate of degradation increases with temperature, and their stability gradually decreases. The higher the temperature, the worse the color development ability [16]. Therefore, turmeric pigments should be stored in the dark at low temperatures.
1.3.2 pH
Turmeric pigment is less stable in strong acid and alkali environments after being dissolved in an ethanol solution. When the pH is less than 5, the turmeric pigment is stable, and as the pH increases, the degradation rate increases significantly [16]. Curcumin exhibits different colors under different pH conditions. It is light yellow in an acidic environment, rose red in a neutral environment, and reddish brown or brown in an alkaline environment. It can also return to yellow after acidification [17]. Therefore, curcumin can be used as a chemical indicator [18].
1.3.3 Metal ions
Certain metal ions such as Fe3+, K+, Fe2+, Al3+, Ca2+ and Cu2+ are prone to chelate with the β-diketone structure in curcumin and precipitate. Moreover, the concentration of metal ions is related to the degree of influence [19-20]. Therefore, contact with aluminium, calcium, iron and copper containers should be avoided during processing, transportation, storage and use [21-22].
1.3.4 Additives
The coloring power and stability of curcumin are affected not only by light, temperature, pH and metal ions, but also by additives such as salt, preservatives, citric acid and vitamin C. Qiao Qingqing et al. [23] dissolved turmeric pigment in ethanol and studied the absorption spectrum of the pigment. They found that the absorbance of turmeric pigment decreased slightly and the color became slightly lighter after the addition of citric acid and vitamin C. The effect of some food additives on curcumin is shown in Table 1 [24].
1.3.5 Other factors
Curcumin has antioxidant and anti-reduction properties, but they are not very strong. It should be avoided from being mixed with strong oxidants (such as H2O2) and strong reducing agents (such as Na2SO3). The type and amount of antioxidant have a relatively large effect on the stability of the curcumin solution [23]. The curcumin solution is relatively unstable in an alkaline environment, and the hydroxyl group is highly reactive and can undergo a variety of reactions.
Curcumin is affected by the above factors. If its instability is not protected and improved, it often fails to achieve the desired goals. Therefore, it is necessary to find ways to improve the stability of curcumin and make it play a better role.

2. Fields of application of curcumin
Turmeric extract is a natural pigment with high practical value and safety [25] and is widely used in food, pharmaceuticals, cosmetics, textiles, animal husbandry and other fields (as shown in Figure 1).
2.1 Food
Turmeric extract is a natural compound with a bright color that can be used as a food additive and preservative [26]. As early as 1981, curcumin was included as a food additive in GB 2760-1981 “Hygienic Standards for the Use of Food Additives”. In 1995, it became a food additive approved by the Food and Agriculture Organization of the United Nations Codex Alimentarius Commission. In the newly published 2011 book “Standards for the Use of Food Additives”, it can be used in moderation according to the type of product and different production needs [27]. Therefore, curcumin is widely used as a food additive and preservative in domestic and foreign foods and beverages [28].
Wu Changling [29] formed a complex with myofibrillar proteins by complexing curcumin, and the embedding efficiency could reach 80%, with a particle size of about 300 nm. The stability of chicken breast meat was also verified, and the structure showed that the complex improved the overall antioxidant properties of chicken breast meat, which can be applied to functional meat products. Xie et al. [30] studied the preparation of porous wheat starch by multiple wet heat treatment combined with complex enzyme hydrolysis, and then encapsulated curcumin. The encapsulation efficiency was about 75%. Compared with unencapsulated curcumin, the stability of encapsulated curcumin in terms of light, heat, and storage time was improved, and it can be used as an encapsulant in food production.

2.2 Medical
Turmeric extract, as a natural pigment, can be used not only in the food industry, but also has many biological activities and pharmacological effects, such as lowering blood pressure and improving blood circulation, relieving depression, clearing heat from the blood, preventing arteriosclerosis, anti-oxidation, anti-inflammatory, anti-tumor, antibacterial, anti-HIV, etc. [31-33].
Shih et al. [34] reported in 1993 that curcumin acts as a free radical scavenger, preventing the formation of 8-hydroxydeoxyguanosine in DNA molecules. Leonid et al. [31] reported in 1996 that curcumin has an antioxidant effect on human red blood cells and their cell membranes. at 4–100 μmol/L can protect human red blood cells from H2O2-induced lysis and lipid peroxidation. Sugimoto et al. [35] treated mice with enteritis with 0.5%, 2.0% and 5.0% curcumin, the study showed that curcumin treatment can prevent and improve intestinal inflammation. Sindhwani et al. [36] reported the inhibitory effect of curcumin on mouse bladder tumor cells. The results showed that 100 μmol/L can significantly inhibit the growth of tumor cells in the mouse bladder.
2.3 Cosmetics
Turmeric extract not only has excellent pharmacological effects, but can also be used as a natural coloring agent in cosmetics. Saraf et al. [37] developed a nano-cream containing curcumin in 2011 that can penetrate dermal cells to produce an anti-wrinkle effect. The results showed that the cream improved the elasticity, firmness, and fatigue of the skin by 30% to 50%. Li Ziyi's research group [38] added curcumin to BB cream, which has a good concealing and brightening effect on the skin without any irritation.
2.4 Textile printing and dyeing
Turmeric extract is non-toxic, safe and environmentally friendly, and can be widely used in the field of textile printing and dyeing. While coloring textiles, turmeric extract's excellent biological activity and pharmacological effects can be used to give textiles functionality. Wang Xuemei's research group [39] used turmeric extract to dye wool, silk, cotton, viscose and hemp fibers, obtaining dyed fabrics with different colors. Peng Wenfang et al. [40] investigated the dyeing properties of curcumin on jute fabrics. When the dyeing time was 90 min, the dyeing temperature was 70 ℃, the dye concentration was 2.5 g / L, the bath ratio was 1 ∶80, and the pH was 3.5, the dyeing rate reached 82.5%, and the dyed fabric had a soft and unique color.

Miao Shuang et al. [41] investigated the foam dyeing of wool fabrics with curcumin. A foam finishing agent was prepared by coupling nitroaniline with curcumin through diazotization, and was directly added to the dyed wool fabric. The optimized process for foam dyeing was 50% finishing agent, 6% foaming agent, temperature 120 °C, time 5 min, and the fabric's light fastness is improved to 2-3. Dong Shuchi et al. [42] found that in addition to dyeing products, turmeric pigment finishing of silk products can also obtain UV resistance, with an UPF value 3-4 times higher.
2.5 Other
Curcumin is also used as a feed additive or encapsulated in microcapsules for use in animal husbandry [43-44]. Zhou Arong et al. [45] combined curcumin with biopolymers to form a film delivery system, which was applied to food packaging materials, freshness monitoring, and antibacterial preservation.
Hu Yuli et al. [46] investigated the conditions for dyeing cow hair with curcumin: dyeing temperature 50 °C, pH 5.0, mordant dosage 3%, dyeing time 80 min. At this time, the dyeing rate is the best and the stability is good. It can also be combined with comfrey. Comfrey turns black and grey, and curcumin turns dark brown, so it can be used in hair dye products. Li Haiming et al. [47] showed that curcumin can be used as a papermaking dye in the paper industry.
3. Improving the application performance of the natural pigment curcumin
3.1. Molecular structure modification
The curcumin molecule contains multiple double bonds, phenolic hydroxyl groups, carbonyl groups, and ketone groups, so it can undergo strong chemical reactions [11]. Sun Xin [48] used diazotization to couple the azo group to the sulfanilic acid diazonium salt to graft new functional groups onto the curcumin molecule to achieve the goal of chemical modification. The modified curcumin (the structural formula is as follows) is easily soluble in water, and the color fastness to soaping of the dyed silk reaches 4 to 5, and the color fastness to sunlight is better than that of pure turmeric dyeing. Wang Zhongyan [49] linked curcumin to a polypeptide to produce a reticular small molecule hydrogel, which improved the water solubility and biocompatibility of curcumin and achieved better anti-cancer effects. Boonroeng [50] introduced a trimethyl ammonium chloride group into the quinoid molecule via an etherification reaction. The modified curcumin produced had better water solubility, dyeability and antibacterial activity, and improved UV resistance. The synthetic route of curcumin and ammonium glycidyl trimethyl chloride is shown in Figure 2a.
Zhou et al. [51] introduced a water-soluble active ultraviolet absorber into the curcumin molecule to prepare a new water-soluble curcumin with good water solubility. It is used in the dyeing and functional finishing of silk fabrics to obtain better color fastness, especially rubbing fastness and light fastness (improved from grade 2 to grade 4), UV resistance, and antibacterial activity (bacteriostatic activity against Escherichia coli and Staphylococcus aureus exceeding 90%). Figure 2b shows the process route for curcumin and UV absorbers. Yuan Bo [52] prepared a molecularly imprinted polymer (MIP) for curcumin using molecular imprinting technology. The bulk polymerization process uses curcumin as a template, EDMA as a cross-linking agent, and AIBN as an initiator, with a mass ratio of 1:4:20. The imprinting factor IF reaches 1.27. The precipitation polymerization process is carried out in a mixed solvent of 1.0 mmol of curcumin, 50 mL of acetonitrile, and 20 mL of methanol. MIP has strong selective adsorption capacity for curcumin, and TG/DTG indicates excellent thermal stability, which can be used in high-temperature environments. Figure 2c and Figure 2e show the molecular imprinting technology preparation process and schematic diagram, respectively.
The above are all based on improving the basic structure of curcumin molecules to overcome application limitations and optimize physical and chemical properties, so that curcumin can be used in a wider range of applications.
3.2 External improvement
3.2.1 Encapsulation
3.2.1.1 Microcapsule Encapsulation
Microcapsule encapsulation, also known as microcapsule technology, refers to the technique of encapsulating (coating or embedding) a functional substance with certain polymeric or inorganic compounds using mechanical, chemical or both methods to form a microcontainer with a core-shell structure and a particle size of 1–500 μm [53]. Micro-sized containers consist of a core material and a wall material. The wall material protects the core material from external interference. Common core materials include dyes, finishing agents, fragrances, fungicides, adhesives, cross-linking agents, catalysts, flame retardants, drugs, nanoparticles, living cells, etc., which can be used alone or in combination. The wall material is formed from natural or semi-synthetic polymer materials with film-forming properties and inorganic compounds such as chitin, alginate, polyester and some surfactants [54-56]. Different core materials require the use of different wall materials for encapsulation, and therefore have the characteristics of diversity, functionality and high efficiency [57]. The performance of microcapsules is closely related to the material of the selected wall material, as well as the microcapsule technology and process method. The properties obtained by different preparation methods also differ.
Microencapsulation technology is a technology that encapsulates solids, liquids, gases, etc. in microcapsules to form a solid particulate product. The core of the capsule is protected from external environmental interference, and then released under appropriate conditions by certain specific means [58-59]. Microencapsulation technology is complex and cumbersome, and can be roughly divided into phase separation (coacervation), polymerization reaction and mechanical methods [60]. The specific technology depends on the nature of the polymer used in the microcapsule and the nature of the coating material. Due to its excellent properties, it has been widely used in food [61], medicine [62], agriculture [63], daily chemicals [64], liquid crystal detector devices, biological products [65], coatings [66], and textiles [67–69] (including textile pretreatment, dyeing, and finishing), and has achieved good social and economic benefits.
Guri et al. [70] studied the stability and cellular uptake of curcumin encapsulated in ultracentrifugation nanoparticles. The encapsulated curcumin can better disperse, protect, enhance delivery and improve stability, and enhance the bioavailability of curcumin. Compared with unencapsulated curcumin, the stability of encapsulated curcumin can be increased to 90% in short-term storage (6 h at 37 °C) or even longer. Zhang Pengfei et al. [71] used modified starch as the wall material to prepare curcumin microcapsules, obtaining finished products with flowing orange-yellow particles. The moisture content was 3.2%, the bulk density was 0.66 g/cm3, the compactness was 0.78 g/cm3, and the average particle diameter was 346.9 nm. The water solubility and stability were improved, The preparation process of the ginger yellow pigment microcapsule particles is shown in Figure 3.
Sun Xiaozhu et al. [72] used β-cyclodextrin as the wall material to prepare ginger yellow pigment microcapsules under the conditions of a core-wall ratio of 1:4, a temperature of 50 °C, a time of 2 h, and an ethanol content of 50%, and dyed polyester. It was found that the equilibrium dyeing rate K/S value could be increased by 15.9, and the color fastness to soap washing could reach grade 4 to 5. Figure 4 shows the process diagram for dyeing ginger yellow pigment on polyester fabric. In addition, many studies have attempted to encapsulate ginger yellow pigment using other delivery systems, such as hydrogels, liposomes and other microcapsule embedding methods. Microencapsulation technology has the excellent properties of improving the water solubility and stability of ginger yellow pigment, extending the storage time, reducing environmental pollution, and changing the compatibility of substances.
The structure type, preparation technology, raw material cost, process procedure, and mechanism of microcapsules are important factors affecting the application and development of microcapsule technology. With the deepening of research, the scope of microcapsule applications is gradually expanding. However, microcapsule technology is complicated and complex, with uncontrollable factors in the preparation technology, limited material selection, insufficient stability of the finished product, poor self-recovery ability, low strength, insufficient use time, poor binding between the microcapsule and the reactant, high raw material and production costs, and an immature process. Moreover, the in vivo reaction mechanism between the microcapsule and the reactant has not yet been fully explored, and there are still many problems that need to be solved urgently. Therefore, there is a need to find more optimized, economical and environmentally friendly technologies.
3.2.1.2 MOF material encapsulation
Metal-organic framework materials (MOFs) are a new type of porous material formed by the self-assembly of metal ions and organic ligands [73]. Among them, metal ions are generally divalent transition metal ions such as Cu2+, Zn2+, Co2+, Pt2+, etc., and the commonly used organic ligands include dimethylimidazole, terephthalic acid, glutaric acid, carboxylic acid, etc. [74]. MOF materials have the characteristics of large specific surface area, high porosity, complete pore structure, adjustable pore channels, and excellent hydrothermal stability and chemical stability [75]. At present, MOF materials have shown excellent performance in gas storage, adsorption and separation [76-78], drug sustained release [79], electrochemistry [80], biosensors [81], biomedical imaging [82], catalysis [83-84], wastewater treatment [85-87] and other applications.
Yifan et al. [88] were the first to propose the use of ZIF-8 porous materials in the protection of natural pigments and the field of textile printing and dyeing in 2021. In the same year, the research group [89] successfully prepared environmentally friendly and stable pigments by physically encapsulating melanin with ZIF-8 porous materials (as shown in Figure 5). Zeolite imidazole framework material (ZIF-8) was used as a carrier and the melanin analog as a payload. 100 mg of ZIF-8 and 12 g/L of melanin analog were coated for 3 h under magnetic stirring at a speed of 2,000 r/min and a temperature of 30 °C. The coating rate was measured to be more than 50% by infrared spectroscopy, and the stability of the pigment was improved.
3.2.2 Adding stabilizers
Turmeric itself contains demethoxycurcumin, which can be used as a natural stabilizer for curcumin to improve the stability of curcumin preparations. Turmeric pigment is encapsulated in β-cyclodextrin, which greatly improves its water solubility and has a stabilizing effect on light, heat and oxidants. Han Xingman et al. [90] used a natural carbohydrate nanoparticle, vegetable glycogen, as a carrier to load turmeric pigment to prepare a complex. After loading on vegetable glycogen, the solubility, ultraviolet stability, acid and alkali stability and biological activity of turmeric pigment were significantly enhanced. Zheng Junhua [91] studied the effect of stabilizers on the stability of curcumin and found that malic acid (1.0:0.5) + citric acid (1.0:0.3) had the best stabilizing effect on the pigment, and the residual rate could reach 88.65% after 10 h of heat preservation.
In addition, other stabilizers such as gum arabic, Zn2+ solution, malic acid, sodium alginate, ascorbic acid, and succinic acid also improve the stability of turmeric yellow pigment. The use of a compound stabilizer is more effective than the use of a single stabilizer [91].
3.2.3 Improving processing and storage conditions
The processing of natural pigments at home and abroad is limited by problems such as unsophisticated processing techniques, simple equipment, and unstandardized processes, which restricts the development of natural pigments [92]. Therefore, the processing conditions of curcumin can be improved by optimizing the process route and improving the equipment level, thereby enhancing the physical and chemical properties. In actual production, the application of technologies such as supercritical fluids, ultrasound (microwave) assistance, membrane separation, chromatography, macroporous adsorption resins, and biological enzymatic hydrolysis has significantly improved the utilization rate of curcumin [93]. The research and use of advanced equipment has also improved the stability of curcumin [94]. Li Shukun et al. [95] added povidone as an excipient to curcumin, which improved the dissolution and stability of curcumin. In short, optimizing the process conditions is very important for the stability of the pigment.
4 Conclusion
At present, due to the disadvantages of curcumin, such as poor stability, easy hydrolysis, and low dyeing efficiency, there are certain problems in its extraction, purification, storage, and use at home and abroad. In order to solve these problems, some methods to improve the stability of natural pigments have been proposed, including microencapsulation technology, the addition of antioxidants, the addition of pigment stabilizers, and the chemical modification of the structural groups of natural pigments. However, there are still deficiencies, and it is necessary to continue to research and explore new methods and technologies. Encapsulation with MOF materials Turmeric yellow pigment is a new method that is simple and easy to implement and can better solve the above problems. The feasibility of encapsulating and protecting natural pigments with MOF materials has been demonstrated. In the future, efforts can focus on screening MOF materials with good physicochemical properties and low toxicity to encapsulate turmeric yellow pigment to improve its stability and dyeing performance, so that turmeric yellow pigment can become a natural dye with good compatibility for coloring textiles. It is expected that this method can also be extended to the protection and application of other natural pigments. It is hoped that in the near future, natural pigment-stabilized environmentally friendly pigments encapsulated in MOF materials can be widely used in the coloring of textiles, food, pharmaceuticals, and cosmetics.
Reference:
[1] ZHOU Y,LU J,ZHOU Y,et al.Recent advances for dyes removal using novel adsorbents: a review[J]. Environmental Pollution, 2019, 252(Part A):352-365.
[2] ABBASI F, TAVAKKOLI Y M, FARROKHNIA A, et al. Keratin nanoparticles obtained from human hair for removal of crystal violet from aqueous solution: optimized by Taguchi method[J].International Journal of Biological acromolecules,2020, 143:492-500.
[3] FERRAZ E R A, LI Z H, BOUBRIAK O, et al. Hepatotoxicity assess⁃ ment of the azo dyes disperse orange 1 (DO1), disperse Red 1 (DR1), and disperse Red 13 (DR13) in HEPG2 Cells[J].Journal of Toxicolo⁃ gy and Environmental Health,2012,75(Part A):991-999.
[4] SHAHID A, MUHAMMAD H, FAZAL-UR R, et al. Microwave-assist⁃ ed sustainable dyeing of wool fabric using cochineal-based carminic acid as natural colorant[J].Journal of Natural Fibers, 2018, 16: 1026-1034.
[5] VELMURUGAN P,KAMALA-KANNAN S,BALACHANDAR V,et al. Natural pigment extraction from five filamentous fungi for industrial applications and dyeing of leather[J].Carbohydrate Polymers,2010,79: 262-268.
[6] ALI S, HUSSAIN T, NAWAZ R. Optimization of alkaline extraction of natural dye from Henna leaves and its dyeing on cotton by exhaust method[J].Journal of Cleaner Production,2009, 17:61-66.
[7] WROLSTAD R E, CULVER C A. Alternatives to those artificial FD&C food colorants[J].Annual Review of Food Science and Technol⁃ ogy,2012,3:59-77.
[8] SOUKOULIS C, BOHN T. A comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids[J].Food Science and Nutrition,2018,58(1):1-36.
[9] LEONG H Y, SHOW L P, LIM M H, et al. Natural red pigments from plants and their health benefits:a review[J]. Food Review Internation⁃ al,2018,34(5):463-482.
[10] BOWLES D, ENGKIAT LIM, POPPENBERGER B, et al. Glycosyl⁃ transferases of lipophilic small molecules[J].Annual Review of Plant Biology,2006,57(1):567-597.
[11] MOHAMMAD M, KARIMI L. Antibacterial dyeing of polyamide us⁃ ing turmeric as a natural dye[J].Autex Research Journal,2013, 13(2): 51-56.
[12] MASUDA T, HIDAKA K, BANDO H, et al. Chemical studies on anti⁃ oxidant mechanism of curcumin: analysis of oxidative coupling prod⁃ ucts from curcumin and linoleate[J].Journal of Agricultural and Food Chemistry,2001,49(5):2539-2547.
[13] Gao Mei . Stability and photodegradation of natural pigments[D]. Suzhou: Soochow University, 2015.
[14] Wang Xuemei, Chen Lihua, Shi Wenting . Study on the photostability of curcuminoid compounds[J]. Journal of Anhui University (Natural Science Edition), 2012, 36(3): 73-78.
[15] Shi Wenting. Extraction, separation and properties of curcuminoid compounds [D]. Hefei: Anhui University, 2011.
[16] Yang Junjun. Extraction, antioxidant activity and stability of curcumin [D]. Changsha: Central South University of Forestry and Technology, 2009.
[17] WANG Y J, PAN M H, CHENG A L, et al. Stability of curcumin in buffer solutions and characterization of its degradation products[J]. Journal of Pharmaceutical and Biomedical Analysis, 1997, 15(12): 1867-1876.
[18] WIMMER M A, GOLDBACH H E.A miniaturized curcumin method for the determination of boron in solutions and biological samples[J]. Journal of Plant Nutrition and Soil Science, 1999, 162(1):15-18.
[19] Wang Xianchun. Study on the stability of curcumin [J]. Food and Fermentation Industry, 1994(1): 63-66.
[20] Zhu Jinshun, Fang Lidan, Fang He. Extraction process and pigment stability analysis of curcumin [J]. China Fiber Inspection, 2015(1): 86-88.
[21] Liu Wei, Ding Ziqing. The effect of several common metal ions and temperature on the stability of curcumin [J]. Food and Fermentation Industry, 1991(2):61-64.
[22] Xiao Bing. Study on the interaction between curcumin and metal ions [D]. Shihezi: Shihezi University, 2017.
[23] Qiao Qingqing, Ren Shuncheng, Lv Zhenzhen. Study on the stability of curcumin [J]. Jiangxi Food Industry, 2011(4):45-48.
[24] Qi Lili, Wang Jinbo. Study on the stability of curcumin [J]. Food Industry Science and Technology, 2007(1):181-182.
[25] Li Xiangzhou, Zhang Yanqiang, Kuang Chuntao. Research progress on the biological activity and extraction and separation of curcumin [J]. Journal of Central South University of Forestry and Technology, 2009, 29(3): 190-194.
[26] Liu Yujia. Research on the interaction of curcumin and biological macromolecules in food [D]. Guangzhou: South China Agricultural University, 2017.
[27] Yuan Peng, Chen Ying, Xiao Fa, et al. Bioactivity of curcumin and its application in food [J]. Food Industry Science and Technology, 2012, 33(14): 371-375.
[28] Ren Erfang, Niu Debao, Xie Chaomin, et al. Progress in the development and application of turmeric [J]. Light Industry Science and Technology, 2014(10):3.
[29] Wu Changling. Study on the construction and application of curcumin-myofibrillar protein complexes under different NaCl concentrations by alkali treatment [D]. Nanjing: Nanjing Agricultural University, 2019.
[30] Xie Ying. Study on the preparation, structural properties and application of wheat porous starch [D]. Hefei: Hefei University of Technology, 2019.
[31] GRINBERG L N, SHALEV O,TØNNESEN H H, et al. Study on cur⁃ cumin and curcuminoids:XXVI. antioxidant effects of curcumin on the red blood cell membrane[J].International Journal of Pharmaceutics, 1996, 132(1/2):251-257.
[32] Luo Peng. Study on the extraction and purification of curcumin in Indonesia [D]. Zhengzhou: Henan University of Technology, 2006.
[33] Han Ting, Mi Heming. Research progress on the chemical composition and pharmacological activity of turmeric [J]. PLA Pharmacy Journal, 2001(2): 95-97.
[34] SHIN C A, LIN J K. Inhibition of 8-hydroxyde oxyguanosine forma⁃ tion by curcumin in mouse fibroblast cells[J]. Carcinogenesis, 1993, 14(4):709-712.
[35] SUGIMOTO K,HANAI H,TOZAWA K,et al. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice[J]. Gastroenterology,2003, 123(6):1912-1922.
[36] SINDHWANI P, HAMPTON J A, BAIG M M, et al. Curcumin pre⁃ vents intravesical tumor implantation of the MBT-2 tumor cell line in C3H mice[J].The Journal of Urology,2001, 166(4):1498-1501.
[37] SARAF S P, JESWANI G, KAUR C D, et al. Development of novel herbal cosmetic cream with Curcuma longa extract loaded transfer⁃ somes for antiwrinkle effect[J].African Journal of Pharmacy and Phar⁃ macology,2011,5(8):1054-1062.
[38] Li Ziyi. Extraction and separation of curcumin and its application in cosmetic products [D]. Guangzhou: South China University of Technology, 2018.
[39] Wang Xuemei, Qi Chenglong. Research on the application of natural dye turmeric in dyeing several common fibers [J]. Dyeing and Finishing Technology, 2013, 35(4): 24-27.
[40] Peng Wenfang, Wu Jinqiao. Study on dyeing properties of turmeric plant dye on jute fabrics [J]. Wool Textile Science and Technology, 2019, 47(6): 50-53.
[41] Miao Shuang, Cui Yongzhu, He Peifeng. Study on the process of foam dyeing of turmeric pigment in wool fabrics [J]. Wool Science and Technology 2019, 47 (10): 51-54.
[42] Dong Shuchi. The influence of molecular structure of natural dyes on ultraviolet resistance [D]. Suzhou: Soochow University, 2012.
[43] Zhang Hong, Xiao Dingfu. The physiological functions of curcumin and its application in aquaculture [J]. Feed Research, 2021, 44(5): 133-136.
[44] Jiang Yang, Lv Haojie, Liu Huanyu et al. Research progress on the biological functions of microencapsulated curcumin and its application in animal husbandry [J]. Feed Research, 2020, 43(12): 143-146. [45] Zhou Arong, Lin Yilin, Qiu Jianqing et al. Research progress on the construction and functional application of curcumin membrane delivery systems [J]. Food Science, 2020, 41(7): 266-274.
[46] Hu Yuli. Application of natural plant pigments and dyes in hair dyes [D]. Changchun: Jilin Agricultural University, 2018.
[47] Li Haiming. Application of natural dyes in papermaking [J]. Papermaking Chemicals, 2008, 20(5): 58-61.
[48] Sun Xin. Research on the chemical modification of the natural dye curcumin and the dyeing properties of the modified dye [D]. Suzhou: Soochow University, 2002.
[49] Wang Zhongyan. Preparation and application of a small molecule hydrogel based on curcumin and polypeptide [D]. Tianjin: Nankai University, 2015.
[50] BOONROENG, SUPANNEE. Study of the improvement of dyeability and other functional properties of curcumin[D]. Hong Kong: Hong Kong Polytechnic University,2016.
[51] ZHOU Y Y,TANG R C.Modification of curcumin with areactive UV absorber and its dyeing and functional properties for silk[J]. Dyes and Pigments,2016, 134:203-211.
[52] Yuan B. Preparation and adsorption performance of curcumin molecularly imprinted polymers [D]. Nanjing: Nanjing Forestry University, 2017.
[53] Wang H M, Fan Y M, Wang L Y. Research progress of oil and fat encapsulation based on microencapsulation technology [J]. Modern Food Science and Technology, 2018, 34(10): 271-280, 195.
[54] Zhu Zegang. Microencapsulation technology opens up new ways for the development of textile dyeing and finishing applications [J]. Shanghai Dyestuff, 2020, 48(3): 35-41.
[55] Chen Bin, Wang Zongkang, Zhang Min, et al. Research progress of microcapsule wall materials [J]. Phosphate Fertilizer and Compound Fertilizer, 2020, 35(8): 50-52.
[56] SANTANA A A,CANO-HIGUITA D M,DE OLIVEIRA R A,et al.In⁃ fluence of different combinations of wall materials on the microen ⁃ capsulation of jussara pulp (Euterpe edulis) by spray drying[J]. Food Chemistry,2016,212:1-9.
[57] Ma Qiong, Wang Jun. Preparation and performance of phase change microcapsules with different core materials [J]. Packaging Engineering, 2016, 37(17): 59-63.
[58] XIAO Z B,LIU H Q,ZHAO Q X,et al.Application of microencapsula⁃ tion technology in silk fibers[J].Journal of Applied Polymer Science, 2022, 139(25/26):e52351.
[59] Tao Ma, Zhe Sun, Xiaojun Zhang. Overview of microcapsule release mechanisms. Modern Pesticides, 2017, 16(5):1-6.
[60] JAMEKHORSHID A, SADRAMELI S M, FARID M.A review of mi⁃ croencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium[J].Renewable and Sustainable Energy Reviews,2014,31:531-542.
[61] CALDERÓN M, EDITH PONCE-ALQUICIRA E.The role of micro⁃ encapsulation in food application[J].Molecules,2022,27(5):1499.
[62] ARAIZA-CALAHORRA A, MAHMOOD A, SARKAR A. Recent ad⁃ vances in emulsion-based delivery approaches for curcumin: from encapsulation to bioaccessibility[J].Trends in Food Science & Tech⁃ nology,2018,71:155-169.
[63] LIU B X, WANG Y, YANG F, et al. Construction of a controlled-re⁃ lease delivery system for pesticides using biodegradable PLA- based microcapsules[J].Colloids and Surfaces B: Biointerfaces,2016, 144:38-45.
[64] VÂNIA I S,JOANA F P,JULIANA F M, et al. Microencapsulation of essential oils:a review[J].Polymers,2022, 14(9):1730.
[65] RUBINI K,BOANINI E,PARMEGGIANI S,et al.Curcumin-function⁃ alized gelatin films: antioxidant materials with modulated physico- chemical properties[J].Polymers,2021, 13(11):1824.
[66] AMIRI S,RAHIMI A.Anticorrosion behavior of cyclodextrins/inhibi⁃ tor nanocapsule-based self-healing coatings[J]. Journal of Coatings Technology and Research,2016, 13(6):1095-1102.
[67] MONLLOR P, CAPABLANCA L, GISBERT J, et al. Improvement of microcapsule adhesion to fabrics[J]. Textile Research Journal, 2010, 80(7):631-635.
[68] MARIA M M S,ESCOBAR G,MARINO PATRICIA ,et al.Aroma fin⁃ ishing of cotton fabrics by means of microencapsulation techniques [J].Journal of Industrial Textiles,2010,40(1):13-32.
[69] CHEN C H, CHIANG C H. Improvement of flame retardant proper⁃ ties of polyurethane composites using microencapsulation technolo⁃ gy[J]. Polymer-Plastics Technology and Engineering, 2019, 58(3): 316-327.
[70] GURI A, GÜLSEREN I, CORREDIG M. Utilization of solid lipid nanoparticles for enhanced delivery of curcumin in cocultures of HT29-MTX and Caco-2 cells[J].Food & Function,2013,4(9):1410- 1419.
[71] Zhang Pengfei, Liu Aiqin, Zhao Hongshan, et al. Preparation and properties of curcumin microcapsules. China Food Additives, 2020, 31(7): 99-104.
[72] Sun Xiaozhu, Wu Zanmin, Xu Baihui. Preparation of turmeric microcapsules and their application in dyeing. Journal of Tianjin Polytechnic University, 2010, 29(5): 57-60.
[73] FEI C V, YAN M P. Recent advancement in metal-organic frame⁃ work: synthesis, activation, functionalisation, and bulk production[J]. Materials Science and Technology,2018,34(9):1025-1045.
[74] ZHU Q L, XU Q. Metal-organic framework composites[J]. Chemical Society Reviews,2014,43(16):5468-5512.
[75] Feng Ailing, Wang Yanni, Xu Rong, et al. Research progress of multifunctional MOFs-based composite materials [J]. Functional Materials, 2018, 49(11): 11061-11070.
[76] ZHAO Y P, YANG H, WANG F, et al. A microporous manganese- based metal-organic framework for gas sorption and separation[J]. Journal of Molecular Structure,2014, 1074:19-21.
[77] Liu Xinyao. Design, synthesis and properties of metal-organic framework materials constructed by multi-core clusters [D]. Changchun: Jilin University, 2021.
[78] MISHRA P, MEKALA S, DREISBACH F, et al. Adsorption of CO2, CO, CH4 and N2 on a Zinc based metal organic framework[J].Separa⁃ tion & Purification Technology,2012,94:124-130.
[79] KE F,YUAN Y P, QIU L G,el al.Facile fabrication of magnetic met⁃ al-organic framework nanocomposites for potential targeted drug de⁃ livery[J].Journal of Materials Chemistry,2011,21(11):3843-3848.
[80] WANG S, BAI J F, XING H, et al. Novel alternating ferro-ferromag⁃ netic two-dimensional (4,4) and photoluminescent three-dimension⁃ al interpenetrating PtS-type coordination networks constructed from a new flexible tripodal ligand as a four-connected node[J]. Crystal Growth & Design,2007,7(4):747-754.
[81] Bai Jianping. Synthesis, structure and properties of porphyrin-based bio-metal-organic frameworks [D]. Guangzhou: Jinan University, 2019.
[82] KERBELLEC N, CATALA L, DAIGUEBONNE C, et al. Luminescent coordination nanoparticles[J]. New Journal of Chemistry, 2008, 32(4):584-587.
[83] YANG Y,YAO H F,XI F G, et al.Amino-functionalized Zr(IV) met⁃ al-organic framework as bifunctional acid-base catalyst for knoeve ⁃ nagel condensation[J]. Journal of Molecular Catalysis A: Chemical, 2014,390:198-205.
[84] Li Rui. Design, synthesis and application research of composite photocatalysts based on metal-organic frameworks [D]. Hefei: University of Science and Technology of China, 2015.
[85] HAQUE E,LEE J E,JANG I T,et al.Adsorptive removal of methyl or⁃ ange from aqueous solution with metal-organic frameworks, porous chromium-benzenedicarboxylates[J].Journal of Hazardous Materials, 2010, 181:535-542.
[86] Bai Shuli, Xue Yaojia, Huang Wenhao, et al. Preparation of CdS/ZIF-8 catalyst and its application in the degradation of dyeing and printing wastewater [J]. Journal of Henan Normal University (Natural Science Edition), 2022, 50(2): 121-128.
[87] Feng Xiaodong. Synthesis and properties of metal-organic framework materials based on curcumin [D]. Shenyang: Northeast Normal University, 2020.
[88] Yi Fan, Wang Xuemei, Hong Guoying, et al. Research progress of ZIF-8 porous materials and their application prospects in printing and dyeing [J]. Dyeing and Finishing Technology, 2021, 43(7): 11-14.
[89] Yi Fan, Wang Xuemei, Hong Guoying, et al. Preparation of environmentally friendly melanin-like pigments coated with zeolite imidazole framework materials [J]. Wool Science and Technology, 2021, 49(12): 39-42.
[90] Han Xingman, Fan Jinling, Wang Pan, et al. Plant glycogen loading improves the stability and bioactivity of curcumin [J]. Food Science, 2020, 41(15): 39-47.
[91] Zheng Junhua, Wang Xiujun, Wang Lifang, et al. Stabilization of curcumin [J]. China Food, 2015, 40(1): 287-291.
[92] Li Zongzhe, Li Deyuan, Su Dan, et al. Research on new developments and development strategies for natural pigment processing [J]. China Food and Nutrition, 2015, 21(2): 39-42.
[93] Cao Yanping, Jiao Qingze. Research progress on the extraction technology of curcumin [J]. China Food Additives, 2010(4): 228-232.
[94] OU C, FU T M, LIU Y, et al. Effect of solvents and preparation meth⁃ ods on physicochemical properties of solid-state curcumin[J]. Latin American Journal of Pharmacy, 2016, 35(9):1930-1937.
[95] Li Shukun, Wang Jing, Tong Meng, et al. Study on the properties and dissolution of curcumin-copovidone co-ground powder [J]. Chinese Herbal Medicine, 2020, 51(23): 5949-5955.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






