Study on Nanoginsenoside Preparion
Ginseng has an important place in traditional Chinese medicine, and its medicinal value was recorded as early as in Shennong's Classic of Materia Medica[1]. Ginsenosides are one of the most important pharmacologically active ingredients in ginseng. They have various pharmacological effects, such as regulating blood sugar [2], anti-tumor [3], anti-inflammatory [4], neuroprotection [5], anti-fatigue [6], etc., but due to their poor water solubility, low bioavailability and poor absorption, they have certain shortcomings in clinical application [7]. In recent years, with the development of nanomedicine technology, ginsenosides, as one of the representatives of natural medicines in the 21st century, have attracted the attention of researchers in the development of nanomedicine delivery systems related to ginsenosides. Therefore, the author reviews the current research on ginsenoside nanomedicines, hoping to provide a reference for the development of related preparations in the future.
1 Introduction
1.1 Ginseng saponins
Since the 1960s, researchers have isolated more than 100 types of ginseng saponins from ginseng. Their chemical structures are composed mainly of aglycones and sugar substituents [8]. Ginseng saponins can be divided into dammarane type and oleanane type according to the differences in aglycones and sugars. According to the position of the sugar group, the dammarane-type ginsenosides can be divided into two types: those with the sugar group attached to the C-3 and C-20 positions of the dammarane ring are referred to as protopanaxadiol type, and the protopanaxatriol type, in which the sugar groups are attached to the C-6 and C-20 positions of the damarane ring. The chemical structures of the various types of ginsenosides are shown in Figure 1 [9-11].
The ginsenosides that have been widely studied are mainly of the damarane type, such as ginsenosides CK, Rb1, Rg3, Rh2, Re, Rh1 and Rg1, etc. The specific chemical structures are shown in Table 1 [11-12]. However, due to the poor membrane permeability of ginsenosides, the oral bioavailability is usually less than 5%, and the efficacy of direct application is not good [7]. Exploring suitable drug delivery systems is an important means of enhancing their medicinal value.

1.2 Nanomedicine delivery systems
The development of nanotechnology has promoted the development of many nanomedicines. Nanomedicines have been widely studied due to their unique pharmacodynamic effects, such as large specific surface area, strong stability, and high drug loading. It has been found that nanomedicine delivery systems can improve drug efficacy by increasing solubility, improving drug stability, promoting drug absorption, regulating drug release, and avoiding immune recognition [13-15]. Targeted modification of nanomedicines can deliver drugs more precisely to pathological sites [16-17], and co-loading multiple drugs with different pharmacological effects can enhance the therapeutic effect and reduce adverse reactions [18-19]. In addition, nanodrugs have been widely studied for the treatment of tumors [20]. Many scholars have put forward supporting theories for the tumor targeting of nanodrugs, such as the EPR effect (Enhanced permeability and retention effect), that is, solid tumor tissue has the properties of high vascular density, poor structural integrity, lymphatic reflux disorders, and high local vascular permeability medium concentrations, making it easier for nanoparticles, liposomes and some macromolecular substances to enter and remain in tumor tissue [21-22]. Preparing ginsenosides as a nano-formulation is an effective way to enhance their therapeutic effect.
2 Ginsenosides as therapeutic drugs
2.1 Micelles
Micelles are ordered aggregates of nanoscale macromolecules formed by self-assembly of amphiphilic molecules [23]. The hydrophobic part of the molecular chain forms the core of the micelle, while the hydrophilic part forms the shell [24]. Amphiphilic molecules can be used to encapsulate poorly soluble drugs to improve drug solubility in water, enhance drug stability, prolong their circulation time in the blood, alter their tissue distribution and 5 enhance the therapeutic effect [25].
Ginsenoside CK is the main metabolite of protopanaxadiol-type saponins and has a significant inhibitory effect on a variety of tumor cells [26]. However, CK has poor water solubility and low bioavailability, which limits its clinical application. Zhang et al. [17] prepared a ginseng saponin CK polymer micelle APD-CK with deoxycholic acid-O-carboxymethylchitosan as the carrier modified with a liver cancer-specific targeting peptide A54.
The modification of the A54 peptide allows APD-CK to selectively target liver cancer cells. The IC50 value of APD-CK after 48 h of action on HepG2 cells is 16.32 μg·mL-1, which is much lower than that of free CK (28.19 μg·mL-1). In addition, the release of drugs in APD-CK is pH-resistant, and is more easily released under acidic conditions. This micelle may also have some lysosome targeting ability. In addition to physical embedding, chemically linking drugs to the carrier is also a common means of preparing drug-loaded micelles. Li et al. [27] used ginsenoside Rh2 and triptolide to double-terminate esterify PEG, successfully prepared a “hydrophobic-hydrophilic-hydrophobic” triblock polymer. This polymer can self-assemble into micelles and be used for the delivery of hydrophobic drugs. It releases drugs under low pH conditions and the action of blood serum hydrolases, and can be used as a stable combined drug delivery system in the treatment of lung cancer.
2.2 Liposomes
Liposomes are tiny double-layered vesicles composed of amphiphilic phospholipids. Cholesterol is embedded in the membrane to enhance stability. Hydrophobic drugs can be encapsulated inside the lipid bilayer, while hydrophilic drugs can be embedded in the hydrophilic layer of the lipid membrane [28]. Compared with polymeric nanoparticles, they have fewer adverse reactions and are biocompatible. At present, the few FDA-approved nanoformulations are mainly liposomes for intravenous injection [29-30].
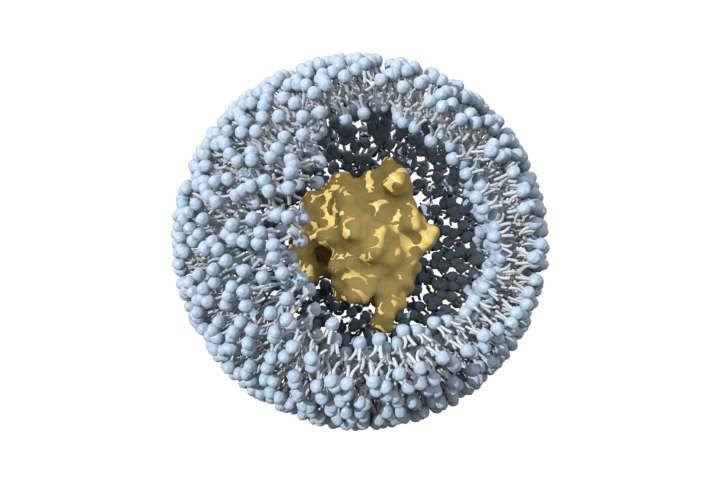
Ginsenoside Rh2 has a good anti-cancer effect in vitro and in vivo, but due to its hydrophobicity and significant efflux mediated by ABC transporters, its bioavailability is very low [31]. Xu et al. [32] prepared mPEG-PLA modified long circulating liposomes Rh2-PLP and and an octadecylamine-modified cationic liposome (Rh2-CLP). Compared with conventional liposome Rh2-LP, both have better physicochemical and biological properties in vitro and in vivo. PLP can target drug delivery to tumor tissue. The fluorescence intensity of DiR-labeled PLP in mouse tumor tissue was 1.3 times that of CLP and 1.6 times that of LP. In vivo anti-tumor studies have shown that Rh2-PLP has the strongest tumor inhibitory effect. This property may be attributed to the PEG modification, which can shield the recognition of liposomes by the reticuloendothelial system (RES), prolong the circulation time of liposomes in the blood, and thus achieve passive targeting of tumors. In addition, other studies have found that the affinity of saponins for cholesterol and phospholipids can affect the structure and formation of liposomes. When the interaction between cholesterol and phospholipids is stronger than that between cholesterol and saponins, it is more conducive to the stability of liposomes [33-34].
2.3 Nanomilks
Nanoemulsions are thermodynamically stable systems composed of an oil phase, a water phase, and a surfactant or co-emulsifier. Strong mechanical forces are usually required to disperse the drug and excipients evenly [35]. However, the preparation process requires less surfactant, the droplet size is small, and the absorption of the drug can be enhanced [36]. It has been studied for the controlled release of bioactive substances when administered orally, by injection, and topically on the skin [37-39].
Ginsenoside Rg1 has low membrane permeability and is not effective when taken orally [40]. In order to improve the oral bioavailability of Rg1, Khattab et al. [41] used Caproyl 90, IPM and Labrafil M1944 as the oil phase, Tween 80, Cremophor EL and Tween 20 as the surfactants, Transcutol HP and propylene glycol as co-surfactants, prepared a Rg1-loaded nanoemulsion SNES. SNES has an ultra-small particle size of 10.05–13.32 nm, which effectively avoids the phagocytosis of RES. The lipid components in the preparation enhance the ability of Rg1 to penetrate the blood-brain barrier, thereby providing higher brain targeting. After oral administration, the metabolites of the lipid components in SNES, such as diacylglycerides, monoglycerides and fatty acids, form mixed micelles with bile acids, which promote the absorption of Rg1 into the blood circulation through the lymphatic system, thereby avoiding the first-pass effect. This nanomilky solution may be an alternative treatment for obese patients with orlistat metabolic disorders.

2.4 Albumin nanoparticles
Albumin is abundant in serum and has the advantages of being non-toxic, non-immunogenic, biocompatible, and easily chemically modified. Based on these properties, albumin nanoparticles have been widely studied for the delivery of various types of molecules, including chemical drugs, proteins/peptides, and oligonucleotides [42-44].
Zhang et al. [45] selected mPEG-SA to esterify the free hydroxyl groups on ginsenoside Rg3 and prepared drug-loaded albumin nanoparticles mPEG-Rg3-BSA NPs using bovine serum albumin (BSA) as the carrier. The introduction of hydrophilic mPEG can significantly prolong the blood circulation of the drug, which is conducive to the mPEG-Rg3-BSA NPs achieving the EPR effect. The fluorescence intensity of DiR-labeled mPEG-Rg3-BSA NPs at the tumor site is 5.4 times higher than that of free DiR. Compared with free Rg3, mPEG-Rg3-BSA NPs enhance the therapeutic effect. Ginsenoside Rg5 has low water solubility and poor bioavailability. Dong et al. [46] used a desolvation method to prepare folic acid (FA) target-modified bovine serum albumin nanoparticles loaded with Rg5 (FA-Rg5-BSA NPs). These nanoparticles specifically target tumor cells due to the large amount of FA on the surface, enhanced nanoparticle uptake and internalization through receptor-mediated endocytosis, inducing apoptosis of tumor cells. In a treatment model of MCF-7 human breast cancer in mice, it was observed that Rg5 could effectively accumulate at the tumor site quickly (8 h), and the inhibition rate of tumor weight in mice reached (79.25±6.36)%, significantly higher than that of free Rg5 (48.84±9.74%) and Rg5-BSA NPs without FA modification (69.91±11.77%).
2.5 Metal nanoparticles
When metal particles are used as drug carriers, the drug can be loaded on the surface of the metal carrier through interactions such as electrostatic forces, hydrogen bonds, and van der Waals forces. In recent years, metal nanoparticles have attracted much attention due to their large specific surface area, ease of functional modification, high stability, and high drug loading capacity. They have been widely studied in tumor imaging, targeted therapy, and photothermal therapy [47-49].
Due to the good biocompatibility and automatic liver targeting function of iron-based nanoparticles, Ren et al. [50] prepared Fe@Fe3O4 nanoparticles Np Rg3 coupled with ginsenoside Rg3, which can significantly inhibit the development of hepatocellular carcinoma (HCC), eliminate the lung metastasis of HCC and effectively prolong the survival of mice with liver cancer. The nanoparticles can also reshape the imbalanced network between intestinal microorganisms and metabolism, delaying HCC-induced intestinal microbial changes for at least 12 weeks, providing a new strategy for the treatment of HCC. In addition, the combination of photothermal therapy and chemotherapy is also an important means of cancer treatment. Kim et al. [51] prepared ginsenoside CK-Au nanoparticles using Lactobacillus acidophilus DCY51T. which have a cationic charge on the surface. When they reach tumor tissue using the EPR effect, they can bind to the anionic surface of tumor cells and invade tumor cells through endocytosis, promoting the lysis of tumor cells. When combined with 635 nm infrared light treatment, the inhibitory effect on the proliferation of human gastric cancer cells is further enhanced, and it is an effective synergistic therapeutic agent for photothermal and chemotherapy.

3 Ginseng saponins as drug carriers
3.1 Ginseng saponins as a drug carrier
The unique chemical structure of ginsenosides makes them valuable as a new type of drug carrier, combining the functions of a carrier and a treatment.
(1) Ginsenosides have both a hydrophobic dammarane or oleanane structure and a hydrophilic glucose moiety[8], which can form self-assembled nanoparticles, micelles or act as surfactants in nanoemulsions[19, 52-54]. (2) Ginseng saponins have a sterol structure and can replace cholesterol as a new type of liposome membrane stabilizer. They have the characteristics of good stability and strong tumor targeting [55-56]. (3) Ginseng saponins can interact with the phospholipids in cell membranes. When used as a drug carrier, they can generate transient gaps when in contact with cell membranes, increasing the cellular uptake of drugs, and the integrity of the cell membrane can be restored in a relatively short period of time [53]. (4) The glycosyl group in the structure of ginsenosides is a substrate for glucose transporter 1 (GLUT1). GLUT1 is specifically expressed in some tumor cells, so ginsenosides themselves also have certain tumor targeting properties [57-58]. (5) GLUT1 is also the main transporter of the blood-brain barrier. Ginseng saponins containing glycosyl groups can also cross the blood-brain barrier, and have certain potential for targeted drug delivery in the brain [56, 59].
(2)
3.2 Application of ginsenosides as drug carriers
3.2.1 Direct formation of self-assembled nanoparticles
Based on the amphiphilic molecular structure of ginsenosides, they can form nanoparticles by themselves and coat other drugs as carriers. Dai et al. [52] used self-assembled ginsenoside Rb1 micelles to deliver low-solubility natural anticancer compounds (betulinic acid, dihydroartemisinin and hydroxycamptothecin). The prepared nanoparticles had a high drug loading capacity (20%–35%), strong tumor targeting and prolonged drug half-life. Li et al. [19] successfully prepared diclofenac-loaded microemulsion ginseng saponin Rb1 micelles with a particle size of less than 10 nm using the thin-film dispersion method. In vivo rabbit ocular corneal penetration studies showed that the micelles could deliver high concentrations of diclofenac to the cornea. After a single dose, the diclofenac levels in the Rb1-Dic administration group were 137.54%, 74.93% and 255.43% higher than those in the commercially available diclofenac ophthalmic solution group at the time points of 0.5, 1 and 2 h, respectively, providing a new strategy for the treatment of eye inflammatory diseases. Zou et al. [53] used ginseng saponin extract as a membrane material prepared ginsenoside-loaded insulin (INS) ginsenoside nanoparticles by thin-film dispersion, which can protect INS from being destroyed by skin hydrolases. The nanoparticles can also rapidly penetrate into cells within 15 minutes by reversibly destroying the intercellular lipid barrier, and based on the skin's reservoir effect, the hypoglycemic efficacy of the formulation in a diabetic rat treatment model remained at about 50% of the initial level for 48 h.
3.2.2 Membrane stabilizer for liposomes
Ginsenoside has a chemical structure similar to cholesterol and can be used as a membrane stabilizer for liposomes instead of cholesterol. Hong et al. [55] prepared liposomes containing paclitaxel using ginsenoside Rh2 and phospholipids, and confirmed that Rh2 has the excellent properties of enhancing the stability of liposomes, prolonging the circulation time of the drug in the blood, promoting the accumulation of the drug in tumors and reversing the immunosuppressive microenvironment. Chen et al. [58] further found that ginsenosides with a C-3-substituted sugar group (such as ginsenosides Rg3 and Rh2) can prolong the blood circulation time of liposomes and specifically bind to GLUT1 expressed on the surface of 4T1 cells, enhancing the targeting ability of the tumor. The sugar residues in the ginsenoside structure are substrates for the glucose transporter of the blood-brain barrier [59]. Zhu et al. [56] used ginsenoside Rg3 as a membrane material to prepare paclitaxel-loaded liposomes Rg3-PTX-LPs, which can also cross the blood-brain barrier for targeted treatment of brain tumors. The IC 50 of Rg3-PTX-LPs for rat C6 glioma cells is 0.045 μg·mL-1, which is much lower than that of cholesterol liposomes C-PTX-LPs (0.149 μg·mL-1). In vivo fluorescence imaging showed that the DiR signal intensity detected in the glioma region of Rg3-LPs was about 3 times that of C-LPs, and it was detected that the elimination of Rg3-LPs in mice was slower than that of C-LPs, and even close to PEG-C-LPs. This type of liposome also has a certain long-circulation effect.

3.2.3 Surfactant as a nanomulsion
Ginsenosides are also a natural surfactant[60] because their chemical structure contains both hydrophobic and hydrophilic groups. They are currently being studied for use in the design and development of new functional foods. Shu et al.[54] dispersed astaxanthin in soybean oil as the oil phase and ginsenosides in high-purity water as the aqueous phase. They successfully prepared nanoemulsions containing astaxanthin by high-pressure homogenization. Even at a relatively low level, ginsenoside can effectively reduce the interfacial tension between the oil and water interfaces. Within a certain concentration range, as the concentration of ginsenoside increases, the droplet size becomes smaller and smaller. However, it is worth noting that when this nanomilky emulsion is stored at higher temperatures, the degradation rate of astaxanthin is too fast, and the specific impact of ginsenoside as a surfactant on the stability of the emulsion needs to be further studied.
4 Conclusion and outlook
Ginsenosides have a variety of pharmacological activities and are natural medicines with great development potential. However, they are highly hydrophobic and have low oral bioavailability, which limits their clinical application. The development of nanodosage forms provides a means of practical application for many poorly soluble drugs. Ginsenosides can be directly encapsulated in nanocarriers such as micelles, liposomes, nanomilks, albumin nanoparticles, and metal nanoparticles, and their bioavailability can be effectively improved. In addition, the hydrophilic sugar group in the hydrophobic ginsenoside structure gives it certain surfactant properties, and it can be used to form nanoparticles or act as a surfactant for nanoemulsions. Furthermore, ginsenoside has a similar structure to cholesterol, and can also be used as a membrane stabilizer for liposomes to enhance their stability. In general, preparing ginsenosides as a nano-formulation can improve their bioavailability. Ginsenosides can also be used as a carrier material to improve the properties of the nano-formulation. When used to encapsulate other drugs, it can also achieve a synergistic therapeutic effect, and has great development value and application potential. However, at this stage, research on ginsenosides as a drug carrier is still in its infancy, and the development of related formulations requires further research.

References:
[1] Sun Xingyan, Sun Fengyi, comp. Shennong's Classic of Materia Medica [M]. Beijing: The Commercial Press, 1955: 9.
[2] SABA, E KIM S H, KIM S D, et al. Alleviation of diabetic complications by ginsenoside Rg3-enriched red ginseng extract in western diet-fed LDL –/– mice[J]. J Ginseng Res, 2018,42(3): 352-355.
[3] ROSA M T M G, SILVA E K, SANTOS D T, et al. Obtaining annatto seed oil miniemulsions by ultrasonication using aqueous extract from Brazilian ginseng roots as a biosurfactant[J]. J Food Eng, 2016,168: 382-390.
[4] Qi B, Zhang S, Guo D, et al. Protective effect and mechanism of ginsenoside Rg1 on carbon tetrachloride induced acute liver injury[J]. Mol Med Rep, 2017, 16(3): 2814-2822.
[5] WANG R, LI Y N, WANG G J, et al. Neuroprotective effects and brain transport of ginsenoside Rg1[J]. Chin J Nat Medicines, 2009,7(4): 315-320.
[6] YANG Q Y, LAI X D, JING O Y, et al. Effects of ginsenoside Rg3 on fatigue resistance and SIRT1 in aged rats[J]. Toxicology, 2018,409: 144-151.
[7] KIM H, LEE J H, KIM J E, et al. Micro-/nano-sized delivery systems of ginsenosides for improved systemic bioavailability[J]. J Ginseng Res, 2018,42(3): 361-369.
[8] CHEN W, BALAN P, POPOVICH D G. Chapter 6 - Comparison of the ginsenoside composition of Asian ginseng (Panax ginseng) and American ginseng (Panax quinquefolius L.) and their transformation pathways[J]. Stud Nat Prod Chem, 2019,63: 161-195.
[9] MOHANAN P, SUBRAMANIYAM S, MATHIYALAGAN R , et al. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions[J]. J Ginseng Res, 2018,42(2): 123-132.
[10] XU X H, LI T, FONG CM V, et al. Saponins from Chinese medicines as anticancer agents[J]. Molecules, 2016,21(10): 1326.
[11] WANG H, ZHENG Y, SUN Q, et al. Ginsenosides emerging as both bifunctional drugs and nanocarriers for enhanced antitumor therapies[J]. J Nanobiotechnol, 2021,19(1): 322.
[12] PIAO X M, ZHANG H, KANG J P, et al. Advances in saponin diversity of panax ginseng[J]. Molecules, 2020,25(15): 3452.
[13] BISWAS S, KUMARI P, LAKHANI P M , et al. Recent advances in polymeric micelles for anti-cancer drug delivery[J]. Eur J Pharm Sci, 2016,83: 184-202.
[14] SHISHIR M R I, XIE L H, SUN C D, et al. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters[J]. Trends Food Sci Tech, 2018,78: 34-60.
[15] DEY T K, KOLEY H, GHOSH M, et al. Effects of nano-sizing on lipid bioaccessibility and ex vivo bioavailability from EPA-DHA rich oil in water nanoemulsion[J]. Food Chemistry, 2018,275: 135-142.
[16] HOON H, HO P M, GAYOUNG J, et al. Injectable glycol chitosan hydrogel containing folic acid-functionalized cyclodextrin-paclitaxel complex for breast cancer therapy[J]. Nanomaterials, 2021,11(2): 317.
[17] ZHANG J M, JIANG YY, LI YP. Micelles modified with achitosan-derived homing peptide for targeted intracellular delivery of ginsenoside compound K to liver cancer cells[J]. Carbohydr Polym, 2020,230: 115576.
[18] SUN J J, LIU Y H, CHEN Y C, et al. Doxorubicin delivered by a redox-responsive dasatinib-containing polymeric prodrug carrier for combination therapy[J]. J Controlled Release, 2017,258: 43-55.
[19] LI M S, LAN J, LI X F, et al. Novel ultra-small micelles based on ginsenoside Rb1: a potential nanoplatform for ocular drug delivery[J]. Drug Delivery, 2019,26(1): 481-489.
[20] GABIZON A A, ROSALES R T M D, LA-BECK N M. Translational considerations in nanomedicine: the oncology perspective[J]. Adv Drug Deliver Rev, 2020,158: 140-157.
[21] GOLOMBEK S K, MAY J N, THEEK B, et al. Tumor targeting via EPR: strategies to enhance patient responses[J]. Adv Drug Deliver Rev, 2018,130: 17-38.
[22] PARK J, CHOI Y, CHANG H, et al. Alliance with EPR Effect: combined strategies to improve the EPR effect in the tumor microenvironment[J]. Theranostics, 2019,9(26): 8073-8090.
[23] DESHMUKH A S, CHAUHAN P N, NOOLVI M N , et al. Polymeric micelles: basic research to clinical practice[J]. Int J Pharm, 2017,532(1): 249-268.
[24] JHAVERI A M, TORCHILIN V P. Multifunctional polymeric micelles for delivery of drugs and siRNA[J]. Front Pharmacol, 2014,5: 77.
[25] AHMAD Z, SHAH A, SIDDIQ M, et al. Polymeric micelles as drug delivery vehicles[J]. RSC Advances, 2014,4(33): 17028–17038.
[26] SHARMA A, LEE H J. Ginsenoside Compound K: insights into recent studies on pharmacokinetics and health- promoting activities[J]. Biomolecules, 2020,10(7): 1028.
[27] LI P, ZHOU X Y, QU D, et al. Preliminary study on fabrication, characterization and synergistic anti-lung cancer effects of self-assembled micelles of covalently conjugated celastrol–polyethylene glycol–ginsenoside Rh2[J]. Drug Delivery, 2017,24(1): 834-845.
[28] DIANA G, ARTUR C P, EUGÉNIAN. Design of liposomes as drug delivery system for therapeutic applications[J]. Int J Pharm, 2021,601: 120571.
[29] DE L V, MILANO F, AGOSTIANOA, et al. Recent advancements in polymer/liposome assembly for drug delivery: from surface modifications to hybrid vesicles[J]. Polymers, 2021,13(7): 1027.
[30] ALMEIDA B, NAG O K, ROGERS K E , et al. Recent progress in bioconjugation strategies for liposome-mediated drug delivery[J]. Molecules, 2020,25(23): 5672.
[31] GU Y, WANG G J, WU X L, et al. Intestinal absorption mechanisms of ginsenoside Rh2: stereoselectivity and involvement of ABC transporters[J]. Xenobiotica, 2010,40(9): 602-612.
[32] XU L Q, YU H, YIN S P, et al. Liposome-based delivery systems for ginsenoside Rh2: in vitro and in vivo comparisons[J]. J Nanopart Res, 2015,17(10): 415.
[33] HAO F, HEY X, SUNY T, et al. Improvement of oral availability of ginseng fruit saponins by a proliposome delivery system containing sodium deoxycholate[J]. Saudi J Biol Sci, 2016,23(1): S113-S125.
[34] GROOT C D, MÜSKEN M, MÜLLER-GOYMANN C C. The bidesmosidic triterpene saponinshederacoside C and ginsenoside Rb1 exhibit low affinity to cholesterol in liposomal membranes[J ]. J Drug Deliv Sci Tec, 2019,53: 101127.
[35] ANTON N, VANDAMME T F. Nano-emulsions and micro-emulsions: clarifications of the critical differences[J]. Pharm Res, 2011,28(5): 978-985.
[36] MCCLEMENTS D J. Advances in edible nanoemulsions: digestion, bioavailability, and potential toxicity[J]. Prog Lipid Res, 2021,81: 101081.
[37] MASKARE R G, INDURWADE N H, DESHMUKH RA , et al. Nanoemulsions: increasing possibilities in oral drug delivery[J]. Asian J Chem Tec, 2021,11(1): 53-58.
[38] SÉGUY L, GROO A C, GOUX D, et al. Design of non-haemolytic nanoemulsions for intravenous administration of hydrophobic APIs[J]. Pharmaceutics, 2020,12(12): 1141.
[39] BASHIR M, AHMAD J, ASIF M, et al. Nanoemulgel, an innovative carrier for diflunisal topical delivery with profound anti-inflammatory effect: in vitro and in vivo evaluation[J]. Int J Nanomed, 2021,16: 1457-1472.
[40] HE C Y, FENG R, SUN Y P, et al. Simultaneous quantification of ginsenoside Rg1 and its metabolites by HPLC– MS/MS: Rg1 excretion in rat bile, urine and feces[J]. Acta Pharm Sin B, 2016,6(6): 593 -599.
[41] KHATTAB A, AHMED-FARID O A, NASR S A. Enhanced brain biodistribution of Ginsenoside Rg1 based self- nanoemulsifying drug delivery system to ameliorate metabolic syndromes and keep homeostatic balance[J]. J Drug Deliv Sci Tec, 2021,61: 1002276.
[42] YHEE J Y, LEE J, CHANG H, et al. Molecular imaging and targeted drug delivery using albumin-based nanoparticles[J]. Curr Pharm Design, 2015,21(14):1889-1898.
[43] LIU Z B, CHEN X Y. Simple bioconjugate chemistry serves great clinical advances: albumin as a versatile platform for diagnosis and precision therapy[J]. Chem Soc Rev, 2016,45(5):1432-1456.
[44] JAIN A, SINGH S K, ARYA S K, et al. Protein nanoparticles: promising platforms for drug delivery applications[J]. ACS Biomater Sci Eng, 2018,4(12):3939-3961.
[45] ZHANG L J, HUI J F, MA P, et al. PEGylation of ginsenoside Rg3-entrapped bovine serum albumin nanoparticles: preparation, characterization, and in vitro biological studies[J]. J Nanomater, 2019: 3959037.
[46] DONG YN, FU R Z, YANG J, et al. Folic acid-modified ginsenoside Rg5-loaded bovine serum albumin nanoparticles for targeted cancer therapy in vitro and in vivo[J]. Int J Nanomed, 2019,14: 6971-6988.
[47] RAI M, INGLE A P, GUPTA I, et al. Bioactivity of noble metal nanoparticles decorated with biopolymers and their application in drug delivery[J]. Int J Pharm, 2015,496(2): 159-172.
[48] GOKUL P, NAMITHARAN K, ARUL M R, et al. Anisotropic noble metal nanoparticles: Synthesis, surface functionalization and applications in biosensing, bioimaging, drug delivery and theranostics[J]. Acta Biomater, 2017, 49: 45-65.
[49] NOOR N A R, BASMA AA, NATHEER H A R, et al. Magnetism in drug delivery: the marvels of iron oxides and substituted ferrites nanoparticles[J]. Saudi Pharm J, 2020,28(7): 876-887.
[50] REN Z G, CHEN X M, HONG L J, et al. Nanoparticle conjugation of ginsenoside Rg3 inhibits hepatocellular carcinoma development and metastasis[J]. Small, 2020,16(2): e1905233.
[51] KIM YJ, PERUMALSAMY H, MARKUS J, et al. Development of Lactobacillus kimchicus DCY51T-mediated gold nanoparticles for delivery of ginsenoside compound K: in vitro photothermal effects and apoptosis detection in cancer cells[J]. Artif Cell, Nanomed, Biotechnol, 2019,47(1): 30-44.
[52] DAI L, LIU K F, SI C L, et al. Ginsenoside nanoparticle: a new green drug delivery system[J]. J Mater Chem B, 2015,4(3): 529-538.
[53] ZOU J J, LE J Q, ZHANG B C, et al. Accelerating transdermal delivery of insulin by ginsenoside nanoparticles with unique permeability[J]. Int J Pharm, 2021,605: 120784.
[54] SHU G F, KHALID N, CHEN Z, et al. Formulation and characterization of astaxanthin-enriched nanoemulsions stabilized using ginseng saponins as natural emulsifiers[J]. Food Chem, 2018,255: 67 -74.
[55] HONG C, LIANG J M, XIA J X , et al. One stone four birds: a novel liposomal delivery system multi-functionalized with ginsenoside Rh2 for tumor targeting therapy[J]. Nano-Micro Lett, 2020,12(10): 73-90.
[56] ZHU Y, LIANG J M, GAO C F, et al. Multifunctional ginsenoside Rg3-based liposomes for glioma targeting therapy[J]. J Controlled Release, 2020,330: 641-657.
[57] RAMANI P, HEADFORD A, MAY M T. GLUT1 protein expression correlates with unfavourable histologic category and high risk in patients with neuroblastic tumours[J]. Virchows Arch, 2013,462: 203 -209.
[58] CHEN C, XIA J X, REN H W, et al. Effect of the structure of ginsenosides on the in vivo fate of their liposomes[J]. Asian J Pharm Sci, 2022, 2022,17(2): 219-229.
[59] WANG Y Z, XU Q, WU W, et al. Brain transport profiles of ginsenoside Rb1 by glucose transporter 1: in vitro and in vivo[J]. Front Pharmacol, 2018,9: 398.
[60] ROSA M T M G, SILVA E K, SANTOS D T, et al. Obtaining annatto seed oil miniemulsions by ultrasonication using aqueous extract from Brazilian ginseng roots as a biosurfactant[J]. J Food Eng, 2016,168: 68 -78.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






