Study on Antioxidant Beta Carotene
Oxidative stress (OS) is a state of imbalance in which the production of reactive oxygen species (ROS) and the clearance of protective mechanisms cannot be offset [1]. An appropriate amount of free radicals helps maintain normal life activities in animals, but when the endogenous clearance system cannot remove excessive free radicals in time, oxidative stress occurs in the body [2]. Oxidative stress can lead to a decline in immune function, chronic inflammation and even organ damage [3]. It can also adversely affect production and reproductive performance, seriously affecting economic efficiency. Beta-carotene is abundant in nature and has extremely high research value for its biological activity. Its function is similar to that of vitamin A and it is a precursor to vitamin A [4].
It is abundant in green to red fruits and vegetables, and the pure product is a shiny dark red hexahedron or crystalline powdery substance. To date, more than 600 natural carotenoids have been discovered. β-Carotene has good antioxidant properties and is a good antioxidant additive. It can reduce organ damage caused by oxidative stress by inhibiting lipid oxidation and promoting the cell's antioxidant defense system [5]. This article mainly outlines the structure, types, sources and metabolism of β-carotene, as well as its antioxidant mechanism of action and application in livestock and poultry production.
1 Structure, types and sources of β-carotene
1.1 Structure of β-carotene
The molecular formula of β-carotene is C40 H56, with a relative molecular weight of about 537. It contains 15 conjugated unsaturated double bonds and two β-ionone rings at both ends. The structure is shown in Figure 1.
The structure of carotene contains multiple conjugated double bonds, which allows it to form multiple cis-trans isomers, including all-trans β-carotene, 9-cis β-carotene, 13-cis β-carotene and 15-cis β-carotene. All-trans β-carotene, 9-cis β-carotene, 13-cis β-carotene and 15-cis β-carotene are some of the more common isomers in nature, with all-trans β-carotene having the highest bioavailability among all isomers [6].
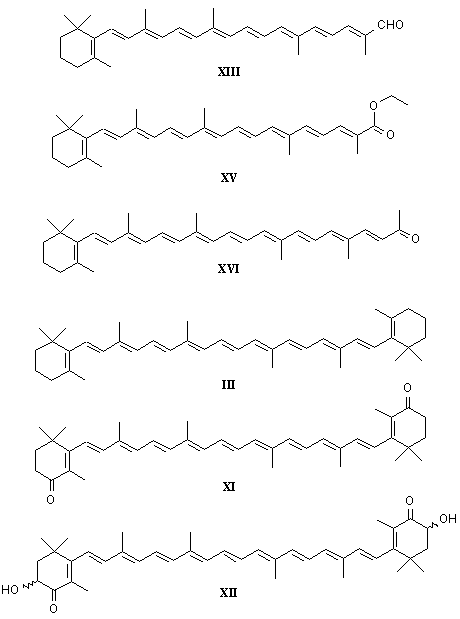
1.2 Types and sources of β-carotene
Beta-carotene can be divided into three main categories based on its source: natural extraction, chemical preparation, and biosynthesis [7]. Extracting β-carotene from natural plants was one of the earliest methods of obtaining β-carotene and played a key role in the early extraction of β-carotene [8]. Natural β-carotene is mainly extracted from fruits and vegetables such as carrots, and microalgae such as Dunaliella.
The chemical synthesis of β-carotene involves the use of precursors with a similar chemical structure to β-carotene, and the synthesis of new β-carotene through multiple chemical reactions under suitable conditions. The chemical formula of the chemically synthesized β-carotene is the same as that of the natural β-carotene, i.e., the chemical formula of the synthetic product is C40 H56, but the structures of the two are not necessarily identical. Carotenoids with different structures have different absorption rates, conversion rates, and solubility in the body, and there are potential, unknown toxic side effects. Therefore, the chemical synthesis of β-carotene has not been widely promoted [9]. The biosynthesis method is mainly used to prepare β-carotene through bacteria, yeasts and other fungi, as well as other genetically engineered bacteria, such as the filamentous fungus Blakeslea trispora [10] and genetically engineered bacteria such as Escherichia coli.
For β-carotene, different preparation methods can affect the color, isomers, particle size in water dispersion and microstructure of β-carotene [11]. Natural carotenoids are safer and more effective during use, but they have problems such as complex processes and low biological activity; the structure and content of each component of chemically prepared β-carotene are different from those of natural substances, and it is unstable during the synthesis process, which leads to greater functional instability [12]; the biosynthesis of β-carotene has the advantages of a short production cycle and easy cultivation, and it is expected that large-scale production of β-carotene can be achieved through microbial synthesis. For a detailed comparison of the three extraction methods, see Table 1.

2 The metabolic mechanism of β-carotene
Beta-carotene is also known as provitamin A because it has the structure of two vitamin A molecules. Due to the existence of a negative feedback regulation mechanism, beta-carotene is converted into vitamin A in livestock and poultry according to their own needs, thus effectively avoiding the problem of excessive accumulation of vitamin A in livestock and poultry [13].
Most of the β-carotene in living organisms exists in the form of a protein complex. The intestine is the main organ for absorbing and converting β-carotene in mammals[14], while the liver is the main organ for storing β-carotene in livestock[15]. There are currently two known metabolic mechanisms for β-carotene in the body. One is the symmetric cleavage reaction that occurs under the action of β-carotene-15,15'-monooxygenase (BCMO1), This reaction is the main pathway for the conversion of β-carotene to vitamin A; the other is an asymmetric cleavage reaction mediated by β-carotene-9',10'-monooxygenase (BCMO1). BCM1 and BCO2 are found in the liver cells and mucosal epithelial cells of the body [14].
BCMO1 has a high degree of selectivity for the chemical bond at the 15,15' position of the carotenoid [16]. Its mechanism of action is to break the double bond at the 15,15' position of β-carotene to form two molecules of retinaldehyde (RAL). Retinaldehyde is then oxidized to retinoic acid (RA) by aldehyde dehydrogenase (ALDH1) or retinal dehydrogenase (RALDH). RA is one of the key metabolites that can reflect the activity of vitamin A [17]. Meanwhile, retinoaldehyde can also be reduced to retinol by alcohol dehydrogenase (ADH) and retinol dehydrogenase (RDH), and some of it is esterified to retinoic acid by retinoic acid ligase (LRAT) and retinoic acid hydroxylase (REH) [14]. The mechanism of action of BCO2 is to cause β-carotene to break at double bonds other than the 15,15' double bond, producing β-ionone and apocarotenoids. Apocarotenoids can be further converted into retinal molecules, but the mechanism is not yet clear. The metabolic process of β-carotene in the body is shown in Figure 2 [18].
BCMO1 also has a unique regulatory mechanism: tissue-specific negative feedback regulation [19]. When the body ingests excess β-carotene or endogenous β-carotene exceeds the scope of its own metabolism, the intestinal epithelial cells secrete a large amount of RA. RA binds to the retinoic acid receptor (RAR) bound to the retinoic acid-specific response element (RARE) in the promoter region of the BCMO1 gene, activating the small intestine-specific homeobox transcription factor (ISX) [20]. ISX suppresses the expression of the BCMO1 gene [21], and can also indirectly suppress the expression of the BCMO1 gene through the high-density lipoprotein receptor (SR-BI) [20, 22], preventing the occurrence of excessive vitamin A accumulation and poisoning [23]. The specific process is shown in Figure 3.
3 Antioxidant activity and mechanism of action of β-carotene
β-carotene has good antioxidant capacity, which can reduce the production of intracellular lipid oxidation and alleviate oxidative stress [24]. It has been found that β-carotene, as a feed additive, has a significant effect on the antioxidant and anticancer properties of livestock and poultry. The current stage of research has found that the antioxidant mechanism of β-carotene may include the following: ① the double bond structure binds to free radicals, reducing their activity or removing them; ② quenching singlet oxygen through electron transfer; ③ promoting Nrf2 mRNA expression, which activates Nrf2 expression and promotes the expression of antioxidant enzymes.
3.1 Scavenging free radicals
Beta-carotene has the ability to scavenge free radicals due to its multiple conjugated polyene double bonds, and is an ideal free radical quencher [25]. Excess free radicals in the body can disrupt the balance of the redox system [26], induce oxidative stress in the animal body, damage cell membranes, cause damage to proteins and DNA, and induce a series of diseases [2]. At present, three reaction pathways have been discovered in which β-carotene eliminates or reduces the activity of free radicals: [27] ① hydrogen atom transfer (HAT), in which the hydrogen atom on β-carotene is transferred to the free radical, thereby weakening the oxidizing effect of the free radical [28]; ② radical addition reaction (RAF), carotenoids directly undergo addition reactions with free radicals to form very stable free radicals [29-30]; and ③ is the electron transfer mechanism (ET), in which the hydroxyl group becomes activated under the influence of β-carotene, promoting hydrogen transfer to the peroxide radical [31]. Wang Ying et al. [32] extracted β-carotene from the fermentation of Trichophora brasiliensis and measured the free radical scavenging rate. The results showed that, at the same temperature, the higher the concentration of β-carotene, the stronger the antioxidant activity of the added substance. Yuan Lei et al. [33] used the DPPH method, salicylic acid method and hydroquinone autoxidation method to determine the ability of four similar but different carotenoids to scavenge free radicals. The analysis showed that β-carotene has the ability to reduce or eliminate free radicals.
3.2. Quenching singlet oxygen
It has been experimentally proven that one molecule of β-carotene can eliminate more than 1,000 singlet oxygen molecules [34]. Since β-carotene is very similar in structure to lycopene and has a similar arrangement of outer electrons, it is possible that β-carotene can quench singlet oxygen by electron exchange energy transfer, that is, electrons from β-carotene and singlet oxygen exchange with electrons from β-carotene with opposite spin directions [35], but this has not been confirmed. By observing the quantitative relationship between β-carotene and singlet oxygen in a single complex, that is, a decrease of 1 to 2 β-carotene in the complex, and a significant increase in the number of singlet oxygen, Telfer et al. concluded that β-carotene can act as an effective quencher of singlet oxygen [36].
3.3 Promoting the expression of antioxidant enzymes
Beta-carotene is a type of non-enzymatic antioxidant in the body that can promote the process by which antioxidant enzymes function [37]. The Keap1-Nrf2-ARE pathway is a key pathway that has been noted in recent years to resist oxidative stress in cells. Nrf2 acts as a switch to activate this signaling pathway [38]. Nrf2 concentration decreases after a period of oxidative stress in the body [39]. β-Carotene has an upregulatory effect on the relative expression of Nrf2 mRNA. The mechanism of action of β-carotene as an antioxidant may be to trigger the Nrf2 “switch” thereby increasing the expression of antioxidant enzyme genes [40]. Rocha et al. [37] demonstrated that β-carotene can change the activity of antioxidant enzymes such as glutathione-S-transferase by analyzing β-carotene nano-dispersions. Sowmya et al. [41] isolated β-carotene from spinach β-carotene can exert an antioxidant effect by observing the expression of antioxidant marker proteins in breast cancer cells (MCF-7), which further explains the anticancer activity of β-carotene.
4 Application of β-carotene's antioxidant properties in the production of livestock and economic animals
In actual production, the antioxidant properties of β-carotene are mainly used to improve animal immunity, improve meat quality, improve growth and production performance, and alleviate the harm of lung and respiratory tract damage and damage to other organs [42]. In addition, β-carotene is non-genotoxic and is recognized as a non-toxic, safe, and nutritious feed additive. When the external environment changes rapidly or when animals are in a special physiological condition, such as pregnancy, they are prone to a series of stress reactions such as oxidative stress. Oxidative stress can cause livestock and poultry to eat less, have decreased resistance, induce various inflammations, and even die, causing certain economic losses to the aquaculture industry. Zhang Xianglun et al. [43] added β-carotene to the diet of beef cattle, and found that the total antioxidant capacity, glutathione content, and total superoxide dismutase activity in the serum of the test cattle were significantly increased, and the malondialdehyde content was reduced, which proved that β-carotene as a feed additive can significantly improve the antioxidant function of beef cattle. When treating bovine infectious keratitis, the therapeutic effect can be significantly improved by supplementing the diet with green forage high in carotene content [44].

Pre-treating porcine intestinal epithelial cells (IPEC-J2) with different doses of β-carotene (25, 50, 100, 150, 200 μmol/L) was found to stimulate an inflammatory response, with the highest cell viability and transmembrane resistance in the treatment group. indicating that β-carotene has the special effect of enhancing the communication between intestinal cells and reducing intestinal damage, and plays a role in maintaining the intestinal health of livestock and poultry [45]. The antioxidant properties of β-carotene, its ability to enhance the body's immunity and strengthen the gap junctions between cells are one of the reasons why β-carotene is widely accepted as an anti-tumor agent. Previous studies have shown that as the concentration of β-carotene increases, the inhibition rate of lipid peroxidation significantly increases [46]. β-carotene can be transformed to be compatible with the lipids in cell membranes, and it can quench free radicals before they cause damage to the body, thus playing a key role in protecting membrane lipids [41].
Bi Yulin et al. [47] added different levels of β-carotene to the feed of beef cattle and quantitatively measured the concentrations of serum glutathione (GSH) and serum malondialdehyde (MDA). They found that as the level of β-carotene added increased, the measured indicators showed significant linear and quadratic changes, proving that β-carotene can greatly affect antioxidant function, change blood physiological indicators and meat quality. Wang Bo [48] found that β-carotene can significantly shorten the time to estrus after calving in dairy cows, promote estrus, increase the conception rate, not only improve the quality of milk, but also reduce the incidence of udder disease in dairy cows. Elomda et al. [49] verified that retinol, a decomposition product of β-carotene, has the ability to promote embryonic development during in vitro culture of rabbit embryos.
5 Summary
As a provitamin A, β-carotene can not only resist oxidation, but also improve the body's immunity, regulate glycolipid metabolism, or be used as a natural coloring agent in feed additives, which can play a certain role in preventing cancer. It also plays an important role in maintaining the normal growth and reproduction of livestock and poultry. Research on the absorption, metabolism and biological activity of β-carotene has become a research hotspot and focus at this stage, and relatively outstanding results have been achieved. However, there are still many issues that require further research and exploration, such as the technology and procedures for the artificial synthesis of β-carotene, the cultivation and breeding of high-yield β-carotene yeast strains, etc. In addition, the potential biological activity of β-carotene and the amount to be added to the feed also need to be further explored.
Reference:
[1] Hussain T,Tan B,Yin Y L,et al.Oxidative stress and inflamma- tion : what polyphenols can do for us? [J].Oxidative Medicine and Cellular Longevity,2016,2016 : 1-9.
[2] Sailaja R P,Kalva S,Yerramilli A,et al.Free radicals and tissue damage: role of antioxidants[J].Free Radicals and Antioxidants, 2011,1 (4) : 2-7.
[3] Yang M, Jia X. The current situation of oxidative stress in livestock and poultry feeding [J]. Feed and Animal Husbandry, 2017 (7): 58-59.
[4] Krinsky N I,Johnson E J.Carotenoid actions and their relation to health and disease[J].Molecular Aspects of Medicine,2005, 26(6) : 459-516.
[5] Esrefoglu M,Akinci A,Taslidere E,et al.Ascorbic acid and beta-carotene reduce stress-induced oxidative organ damage in rats[J].Biotechnic & Histochemistry,2016,91 (7) : 455-464.
[6] Khoo H E,Nagendra P K,Kong K W,et al.Carotenoids and their isomers : color pigments in fruits and vegetables[J].Mole- cules,2011,16(2) : 1710-1738.
[7] Chen Yashu, Wang Rong, Xie Bijun, et al. Optimization of extraction conditions for carotenoids produced by Rhodococcus sp. B7740 and identification of methylnaphthoquinone carotenoids [J]. Food Science, 2016, 37(2): 25-30.
[8] Armstrong G A,Hearst J E.Carotenoids 2 : Genetics and molecu- lar biology of carotenoid pigment biosynthesis[J].The FASEB Journal,1996,10(2) : 228-237.
[9] Sheng Baowei, Yang Yigong. Research progress on the fermentation of natural β-carotene [J]. World of Biotechnology, 2014, 11 (9): 62.
[10] Mata-Gómez L,Montaez J,Méndez-Zavala A,et al.Biotechno- logical production of carotenoids by yeasts : an overview[J]. Microbial Cell Factories,2014,13( 1) : 12.
[11] Zhang Lihua, Xu Xinde, Lv Hongping, et al. Effect of different preparation methods on the color, isomers and particle size of β-carotene microcapsule products [J]. Food Industry Science and Technology, 2016, 37(8): 163-166, 170.
[12] Sun Dandan, Li Ming, Kuang Jinmei. Discussion on the use and safety of carotenoids in livestock and poultry [C] ∥ Proceedings of the 2013 Annual Conference of the Chinese Society of Animal Science and Veterinary Medicine. Beijing: Chinese Society of Animal Science and Veterinary Medicine, 2013: 83.
[13] Mei Suhuan, Geng Yue. Research progress on the safety of astaxanthin and β-carotene [J]. Journal of Food Safety and Quality Testing, 2018, 9(19): 5153-5158.
[14] Li Yanqiang. Analysis of β-carotene metabolism in pigs and its effect on piglet growth performance [D]. Changchun: Jilin Agricultural University, 2015.
[15] Reynoso C R , Mora O,Nieves V,et al. β-Carotene and lutein in forage and bovine adipose tissue in two tropical regions of Mexico[J].Animal Feed Science and Technology,2004,113 ( 1) : 183-190.
[16] Kim Y S,Oh D K.Substrate specificity of a recombinant chick- en β-carotene 15,15'-monooxygenase that converts β-carotene into retinal[J].Biotechnology Letters,2009,31 (3) : 403-408.
[17] Kumar S,Dollé P,Ghyselinck N B,et al.Endogenous retinoic acid signaling is required for maintenance and regeneration of cornea[J].Experimental Eye Research,2017,154(2) : 190-195.
[18] Von Lintig J,Sies H.Carotenoids[J].Archives of Biochemistry and Biophysics,2013,539(2) : 99-101.
[19] Van Helden Y G J,Heil S G,van Schooten F J,et al.Knockout of the Bcmo1 gene results in an inflammatory response in female lung,which is suppressed by dietary beta-carotene[J].Cellular and Molecular Life Sciences,2010,67(12) :2039-2056.
[20] Seino Y,Miki T,Kiyonari H,et al.Isx participates in the main- tenance of vitamin A metabolism by regulation of β-carotene 15,15'-monooxygenase ( Bcmo1 ) expression[J].Journal of Biological Chemistry,2008,283(8) : 4905-4911.
[21] Lobo G P,Amengual J,Li H N M,et al. β , β-carotene decreases peroxisome proliferator receptor γ activity and reduces lipid storage capacity of adipocytes in a β, β-carotene oxygenase 1-dependent manner [J].Journal of Biological Chemistry,2010,285(36) : 27891-27899.
[22] Lobo G P,Hessel S,Eichinger A,et al.ISX is a retinoic acid- sensitive gatekeeper that controls intestinal β , β-carotene ab- sorption and vitamin A production[J].The FASEB Journal, 2010,24(6) : 1656-1666.
[23] Lietz G,Lange J,Rimbach G.Molecular and dietary regulation of β, β-carotene 15,15'-monooxygenase 1 ( BCMO1) [J].Ar- chives of Biochemistry and Biophysics,2010,502( 1) : 8-16.
[24] Upritchard J E,Schuurman C R W C,Wiersma A,et al.Spread supplemented with moderate doses of vitamin E and carotenoids reduces lipid peroxidation in healthy,nonsmoking adults[J].The American Journal of Clinical Nutrition,2003, 78(5) : 985-992.
[25] Elvira-Torales L I,García-Alonso J,Periago-Castòn M J.Nutri- tional importance of carotenoids and their effect on liver health : a review[J].Antioxidants,2019,8(7) : 229.
[26] Kawata A,Murakami Y,Suzuki S,et al.Anti-inflammatory activity of β-carotene,lycopene and tri-n-butylborane,ascavenger of reac- tive oxygen species[J].In Vivo,2018,32(2) :255-264.
[27] Zhang Chengyue, Deng Zeyuan, Li Hongyan. Carotenoid scavenging superoxide anion and hydrogen peroxide free radical activity [J]. Journal of Nanchang University (Science Edition), 2018, 42 (2): 129-133.
[28] El-Agamey A,Lowe G M,McGarvey D J,et al.Carotenoid rad- ical chemistry and antioxidant / pro-oxidant properties[J].Ar- chives of Biochemistry and Biophysics,2004,430( 1) : 37-48.
[29] Kumar N,Shukla P K,Mishra P C. Reactions of the OOH radi- cal with guanine : Mechanisms of formation of 8-oxoguanine and other products[J].Chemical Physics,2010,375 ( 1 ) :
118-129.
[30] El-Agamey A,McGarvey D J.Evidence for a lack of reactivity of carotenoid addition radicals towards oxygen : a laser flash photolysis study of the reactions of carotenoids with acylperoxyl radicals in polar and non-polar solvents[J].Journal of the A- merican Chemical Society,2003,125( 11) : 3330-3340.
[31] Zeeshan M,Sliwka H R , Partali V,et al.Electron uptake by classical Electron donators : astaxanthin and carotenoid alde- hydes[J].Tetrahedron Letters,2012,53(34) : 4522-4525.
[32] Wang Ying, Qu Huiming, Zhao Bo, et al. Kinetic study on the antioxidant activity of β-carotene produced by Penicillium triacum [J]. Food Research and Development, 2018, 39 (10): 7-11.
[33] Yuan Lei, Liu Xiaogeng, Tang Yu. Comparison of the free radical scavenging ability of different carotenoids [J]. Packaging and Food Machinery, 2015, 33(2): 7-11.
[34] Siems W,Wiswedel I,Salerno C,et al. β-carotene breakdown products may impair mitochondrial functions : potential side effects of high-dose β-carotene supplementation [J].The Journal of Nutritional Biochemistry,2005,16(7) : 385-397.
[35] Zhang Yingyuan. DFT investigation of the mechanism of lycopene scavenging singlet oxygen [D]. Xinxiang: Henan Normal University, 2017.
[36] Telfer A,Dhami S,Bishop S M,et al.Beta-carotene quenches singlet oxygen formed by isolated photosystem Ⅱ reaction cen- ters[J].Biochemistry,1994,33(48) : 14469-14474.
[37] Rocha F,Sugahara L Y,Leimann F V,et al.Nanodispersions of beta-carotene : effects on antioxidant enzymes and cytotoxic properties[J].Food & Function,2018,9(7) : 3698-3706.
[38] Lu M C,Ji J N,Jiang Z Y,et al.The Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target : an update[J].Medicinal Research Reviews,2016,36 ( 5 ) :924-963.
[39] Sahin K,Orhan C,Yazlak H,et al.Lycopene improves activation of antioxidant system and Nrf2 / HO-1 pathway of muscle in rainbow trout ( Oncorhynchus mykiss) with different stocking densities[J].Aquaculture,2014,430 : 133-138.
[40] Qu Weiming, Wang Ying, Zhao Bo, et al. Protective effect of β-carotene on H2O2-induced liver damage in zebrafish [J]. Food Science, 2019, 40 (5): 162-166.
[41] Sowmya S G,Yogendra P K,Arpitha H S,et al. β-carotene at physiologically attainable concentration induces apoptosis and down-regulates cell survival and antioxidant markers in human breast cancer ( MCF-7) cells[J].Molecular and Cellular Bio- chemistry,2017,436( 1) : 1-12.
[42] Yang M, Jia X. The current situation of oxidative stress in livestock and poultry feeding [J]. Feed and Animal Husbandry, 2017 (7): 58-59.
[43] Zhang X, Fan X, You W, et al. The effect of dietary β-carotene supplementation on the antioxidant capacity of beef cattle [J]. Shandong Agricultural Science, 2017, 49 (6): 119-122.
[44] Dong Hongwei, Wu Min, Zhao Yanli, et al. Research on the effect of β-carotene on the tight junction of intestinal epithelial cells [J]. Heilongjiang Animal Husbandry and Veterinary Medicine, 2016, 59 (2): 109-110.
[45]Li R , Yang Y,Hong P,et al. β-carotene attenuates weaning-in-cuced apoptosis viainhibition of PERK-CHOP and IREl-JNK / p38 MAPK signalling pathways in piglet jejunum[J].Journal of Animal Physiology and Animal Nutrition,2020,36 ( 22) : 246-251.
[46] Sun Xiejun, Pan Longfei, Li Xiuxia, et al. Study on the extraction of β-carotene from salt algae and its free radical scavenging ability [J]. Food Industry Technology, 2015, 36 (22): 246-251.
[47] Bi Yulin, Wan Fachun, Jiang Shuzhen, et al. Effects of β-carotene on beef cattle performance, antioxidant function, blood physiological indicators and meat quality [J]. Journal of Animal Nutrition, 2014, 26(5) : 1214-1220.
[48] Wang B. The role of β-carotene in dairy cow feeding [J]. Modern Animal Husbandry Technology, 2015, 43(1) : 34.
[49] Elomda A M,Saad M F,Saeed A M,et al.Antioxidant and de- velopmental capacity of retinol on the in vitro culture of rabbit embryos[J].Zygote,2018,26(4) : 326-332.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






