Research on the Structure, Performance, Modification and Application of Hyaluronic Acid
Hyaluronic acid (hyaluronan, hyaluronic acid, HA) is a glycosaminoglycan that occurs naturally in living organisms. It was first isolated from the vitreous humor of cattle in 1934 by Karl Meyer and John Palmer of Columbia University in the United States. They named it “hyaluronic acid”, which comes from the words “hyalo-oid” and “uronic acid” [1]. Later, Endre Balazs coined the term “hy aluronan” in 1986 to name hyaluronic acid in line with the international naming convention for polysaccharides, to cover various molecular forms (including acid and salt forms) [2]. Hyaluronic acid is an important component of the cell matrix and various tissues, and has a variety of important physiological functions, such as regulating cell proliferation, migration and differentiation; natural moisturizing; lubricating joints to protect cartilage; regulating protein synthesis; regulating inflammatory responses; regulating immune function; promoting wound healing, etc.
Hyaluronic acid's unique viscoelasticity, biocompatibility and degradability have led to its wide application in the biomedical field, including as an ophthalmic surgical aid, an anti-adhesion agent after surgery, a wound healing and regeneration aid, a drug carrier, a tissue engineering scaffold, etc. This article describes the structure, properties and chemical modification methods of hyaluronic acid, and discusses the current status of its application in the biomedical field. Hyaluronic acid's unique structural characteristics and excellent properties mean that it has extremely promising applications in the biomedical field. The aim of this review is to raise researchers' interest in hyaluronic acid by providing a comprehensive account of it, and to provide some guidance for the design of novel hyaluronic acid biomedical materials.
1 Structure, properties and physiological functions of hyaluronic acid
1. 1 Chemical structure of hyaluronic acid
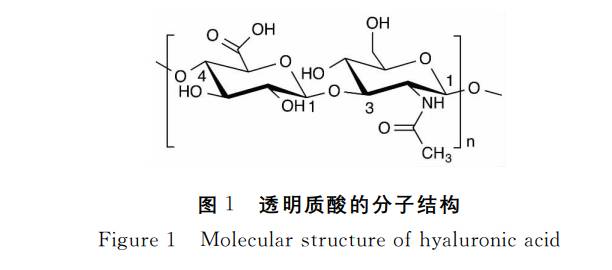
Hyaluronic acid is a member of the glycosaminoglycan (also known as mucopolysaccharide) family. Like other glycosaminoglycans, hyaluronic acid is a high-molecular-weight linear polysaccharide composed of repeating disaccharide units of aminohexose and hexuronic acid. However, it is the only non-sulfated glycosaminoglycan and the only glycosaminoglycan that is not covalently linked to nuclear proteins to form proteoglycans. Unlike most glycosaminoglycans, hyaluronic acid is synthesized on the cell membrane via membrane proteins, rather than via the cell's Golgi apparatus[3]. The disaccharide unit of natural hyaluronic acid is composed of D-glucuronic acid and N-acetyl-D-glucosamine, which are linked by a β-1, 3 glycosidic bond, and the disaccharide unit is linked by a β-1, 4 glycosidic bond, i.e. [(1→ 3)-β-D-GlcNAc-(1→ 4)-β-D-GlcUA-] (see Figure 1), with a molecular weight of up to 10 7 Da [4]. Both sugars adopt the β-configuration, with their hydroxyl, carboxyl, acetamido and hydroxymethyl groups in e-bonding positions, making hyaluronic acid very stable energetically.
1. 2 Properties of hyaluronic acid
Hyaluronic acid is a white, amorphous solid with no smell. It is highly hygroscopic, soluble in water but insoluble in organic solvents. The hydrophilic groups in the molecular structure of hyaluronic acid are all in the parallel positions of the sugar rings, while the hydrophobic hydrogen atoms form a hydrophobic region in the axial direction. Due to the hydrogen bonding between the monosaccharide molecules in the molecular chain, the hyaluronic acid molecular chain forms a rigid columnar helical structure in space. In an aqueous solution, the hyaluronic acid molecules form an expanded, random coil structure. At lower concentrations, these hyaluronic acid chains also entangle with each other to form a continuous three-dimensional network structure with unique rheological properties. Water molecules are fixed in the network formed by hyaluronic acid molecules through hydrogen bonds and are not easily lost. Studies have shown that hyaluronic acid can adsorb about 1000 times its own weight in water, making it the best natural water-retaining substance found in nature. A 1% solution can form a gel, but it is easily fluid under pressure and can pass through the narrow passage of an injection needle. It is a pseudoplastic material. The extraordinary rheological properties of hyaluronic acid solutions make them ideal lubricants, capable of separating the surfaces of most tissues and allowing them to slide along each other.
1. 3 Degradation of hyaluronic acid
The degradation of hyaluronic acid in the body can be seen as a depolymerization process in which glycosidic bonds break, mainly through enzymatic hydrolysis and free radical degradation. The enzymatic degradation of hyaluronic acid in the body is mainly carried out by the hyaluronidase family, which has six members: HYAL-1, HYAL-2, HYAL-3, HYAL-4, HYAL-P1 and PH-20 [5]. Among them, the two most active enzymes are HYAL-1 and HYAL-2. HYAL-2 (located on the cell membrane) cleaves high molecular weight HA (>1MDa) into fragments of 20kDa. HYAL-1 (located in lysosomes) then cleaves these fragments into tetroses, which are further converted into monosaccharides by the action of other enzymes (e.g. β-glucuronidase, β-N-acetylglucosaminidase). Since these degradation products are natural substances that are present in the human body, they can participate in the body's own removal process. On the other hand, free radicals produced by tissue inflammation, etc., also cause oxidative degradation of hyaluronic acid by cleaving the glycosidic bond. The catabolism of hyaluronic acid occurs in situ (e.g., in the extracellular matrix), intracellularly, and in lymph nodes. Long-chain hyaluronic acid is degraded in situ by enzymes and free radicals to produce smaller hyaluronic acid oligosaccharides. These oligosaccharides are then further metabolised inside the cells and lymph nodes, and eventually enter the circulatory system, where they are eliminated by the liver and kidneys [6].
1. 4 Physiological functions of hyaluronic acid
Hyaluronic acid is an important component of the extracellular matrix. In the past, hyaluronic acid was considered to be a simple space-filling substance, and it was only gradually that its importance was recognized. Due to its high water absorption, the primary role of hyaluronic acid in the human body is structural support and moisture retention. It provides lubrication and shock absorption for cells and other extracellular matrix components (including collagen and elastin), while regulating the water balance of tissues and providing a favorable environment for cell migration and proliferation. Hyaluronic acid also has a large number of negatively charged carboxyl groups on its backbone, which act as ion exchangers and can regulate the concentration of cations around cells. In addition, hyaluronic acid also acts as a signaling molecule, participating in cell signaling and regulating various cell activities, including cell proliferation, migration, differentiation, and adhesion, by binding to various protein receptors on the extracellular matrix and cell membrane. Thus, it plays a role in regulating physiological functions of the body, For example, hyaluronic acid can promote the aggregation of white blood cells at the site of inflammation through binding to the CD44 receptor, thereby promoting the body's immune anti-inflammatory effect [7].
This signal-regulating effect of hyaluronic acid is related to its molecular weight, with hyaluronic acids of different molecular weights triggering different signal pathways. High molecular weight hyaluronic acid exhibits anti-angiogenic, scar-inhibiting and anti-inflammatory effects, while low molecular weight hyaluronic acid (<100kDa) exhibits the opposite effects, promoting inflammation, immune stimulation, scar formation and angiogenesis [8]. The cause of this difference is still uncertain. One hypothesis is that high molecular weight hyaluronic acid has the effect of aggregating receptor proteins on cell membranes, while low molecular weight hyaluronic acid does not have this effect [9], thus causing differences in receptor activity and resulting in different physiological functions.
Hyaluronic acid is an intelligent moisturizing factor that can adjust its water absorption according to the relative humidity of the surrounding environment, regulating the water balance of cells and tissues. In the skin, these highly moisturizing hyaluronic acids form an extracellular colloidal matrix with a high water content together with collagen and elastin, giving the skin resilience and elasticity. At the same time, hyaluronic acid also has the effect of scavenging free radicals. As mentioned above, free radicals can oxidize and degrade hyaluronic acid, and hyaluronic acid uses this degradation reaction to remove free radicals in the body through its own rapid metabolism.
Hyaluronic acid is also the main component of synovial fluid, and its high viscoelasticity plays a vital role in protecting joints. It is a viscous liquid at low impact frequencies such as walking, which reduces friction between tissues; an elastic liquid at high impact frequencies such as running, which cushions the impact of stress; and a gel-like elastomer under load, which acts as a cushion to reduce the pressure on the joints[10].
Hyaluronic acid also plays a role in promoting tissue wound healing and is a recognized major compound in this process. It plays an important role in the activation and regulation of immune responses, the promotion of angiogenesis, and cell proliferation and migration. During the inflammatory phase, high molecular weight hyaluronic acid increases, absorbing water to expand and produce a porous scaffold suitable for cell migration, inhibiting the migration of neutrophils, and reducing the inflammatory response. During the proliferation phase, hyaluronan oligosaccharides promote angiogenesis and the migration of fibroblasts to the wound tissue, where they construct a new extracellular matrix. During the reconstruction phase, hyaluronic acid regulates scar formation [11].
2 Industrial production of hyaluronic acid
Hyaluronic acid is widely found in the cell matrix and lubricating fluid of various tissues in animals, including human umbilical cords, joint synovial fluid, skin, thoracic lymphatic fluid, vitreous humor, and rooster combs. The rooster comb is currently the animal tissue found to have the highest hyaluronic acid content (see Table 1) [4]. The extraction process of hyaluronic acid generally involves a complete set of processes such as homogenization, extraction, precipitation, and impurity removal of these freshly collected hyaluronic acid-rich tissues to finally obtain hyaluronic acid with high purity. Although the extraction method has a simple process flow, it is restricted by the limited source of raw materials, low efficiency, and high cost, and has gradually been replaced by the fermentation method.

The use of microbial fermentation to prepare hyaluronic acid first appeared in the 1970s, but it was not until 1985 that Shiseido in Japan first reported the use of Streptococcus fermentation to produce hyaluronic acid. This led to the development of the biological fermentation method, which gradually replaced the traditional animal tissue extraction method and has become the mainstream international method of hyaluronic acid production today [12]. Currently, the commercially produced hyaluronic acid strains include Streptococcus and Bacillus subtilis.
3 Chemical modification of hyaluronic acid
The residence time of pure hyaluronic acid in the human body is relatively short, with a half-life of less than 24 hours after injection into the skin or joints[13] . This greatly limits its application in the biomedical field. However, hyaluronic acid can be further chemically modified due to its multiple active groups, including carboxyl groups, hydroxyl groups, and amino groups exposed by deacetylation, which can give it better mechanical strength, rheological properties, and resistance to enzymatic hydrolysis, etc., thus expanding its scope of biomedical applications.
3.1 Carboxyl modification
3.1.1 Amidation reaction
The carboxyl group of hyaluronic acid can be activated by carbodiimides, 2-chloro-4,6-dimethyl-1,3,5-triazine (CDMT), 2-chloro-1-methyliodopyridine (CMPI), 1, 1,1'-carbonyl diimidazole (CDI), etc. are activated [14~17] and then efficiently reacted with amino compounds to form amide bonds (see Figure 2). Among them, the most widely used activator is 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC). The reaction mechanism is as follows: first, the activated carboxyl group of EDC forms the O-acetyl isourea intermediate, and then the amino group carries out a nucleophilic attack to form an amide bond. Since the O-acetyl isourea intermediate is also prone to rapid rearrangement in reaction with water to form the stable by-product N-acetylurea, in order to prevent the formation of N-acetylurea, N-succinimide (NHS) or hydroxybenzotriazole (HOBT) is added during activation to form a stable, hydrolysis-resistant intermediate (see Figure 3) [18].
On the other hand, the optimum pH for the EDC activation reaction is 3.5~4.5, and amino groups have a high pK a value. Under these pH conditions, the nucleophilicity of protonated amino groups is reduced, and their reactivity with activated carboxyl groups is also reduced. Replacing amino groups with hydrazides with a low pK a (pK a ≈2~3) can increase the reactivity [19]. Hyaluronic acid gels prepared with dihydrazides as cross-linking agents have stronger mechanical properties. When an excess of adipoyl dihydrazide (ADH) is used to react with hyaluronic acid, only a monofunctionalization reaction occurs, forming a stable hyaluronic acid-ADH derivative that retains the other hydrazide as a reaction site for further functionalization (see Figure 2). In practice, many hyaluronic acid-drug precursors are formed by the reaction of the hyaluronic acid-hydrazide intermediate with imine-activated drugs rather than by the reaction of hyaluronic acid itself, because the main by-product when hyaluronic acid is used directly is N-acetylurea.
3. 1. 2 Esterification
In addition to reacting with amino compounds, the carboxyl group of hyaluronic acid can also undergo an esterification reaction with fatty or aromatic alcohols. The above-mentioned activating reagents can also be used to catalyze the esterification of the hyaluronic acid carboxyl group. The activated hyaluronic acid can also undergo a crosslinking reaction with its own hydroxyl groups to form a self-crosslinking gel (the crosslinking structure does not contain a crosslinking agent). In addition to esterification with alcohols, hyaluronic acid can also react with haloalkanes and epoxides to form ester bonds (see Figure 4) [20 , 21] .
3. 2 Hydroxyl modification
3. 2. 1 Etherification
Due to the presence of hyaluronidase, the half-life of natural hyaluronic acid in the human body is relatively short. Therefore, various hyaluronic acid fillers on the market generally use chemical cross-linking to improve their resistance to enzymatic hydrolysis and prolong their retention time in the body. In 1964, Laurent et al. [22] first reported the cross-linking reaction of hyaluronic acid. They used 1,2,3,4-diepoxbutane as the cross-linking agent, and the reaction occurred under strong alkaline conditions with a pH of 13-14. Currently, the cross-linking agents used by major manufacturers around the world include 1,4-butanediol diglycidyl ether (BDDE), 1,2,7,8-diepoxyoctane (DEO), divinyl sulfone (DVS), etc. (see Figure 5) [23] , are mainly used to cross-link hyaluronic acid through etherification. Etherification generally takes place under strongly alkaline conditions. Here, the hydroxyl groups undergo deprotonation (pK a ≈ 10) to form strongly nucleophilic oxygen anions, which preferentially add nucleophilically to deprotonated carboxyl groups to form ether bonds. Under acidic conditions (pH 2~4. 5), the deprotonation of the hydroxyl group is reduced, and the ester bond is mainly formed by the attack of the negatively charged carboxyl group on the epoxy group (see Figure 5) [24]. However, Tomihata and Ikada [25] found that under weak acidic and neutral conditions (pH = 4.7, 6.1, 8.0), the product is still dominated by ethers.
3. 2. 2 Esterification
The hydroxyl groups of hyaluronic acid can also undergo esterification reactions with activated carboxylic acids, anhydrides, and active groups such as acid chlorides. For example, Coradini et al. [26] reported the use of butyric anhydride to react with the hydroxyl group on the trimethylpyridine salt of hyaluronic acid in the presence of pyridine or dimethylaminopyridine to form a hyaluronic acid-butyric acid precursor. This hyaluronic acid-butyric acid precursor drug not only retains the original pharmacological effects of butyric acid, but also promotes the uptake of butyric acid by cells and improves the effect of butyric acid in inhibiting the growth of tumor cells. In fact, hyaluronic acid-butyric acid is completely endocytosed into MCF-7 human breast cancer cells under the mediation of the CD44 receptor, showing relatively obvious tumor targeting.
3. 2. 3 Other reactions
The hydroxyl groups of hyaluronic acid can also undergo other reactions, such as a cross-linking reaction with glutaraldehyde to form a hemicalix[26]. This reaction requires acidic conditions to activate the aldehyde group and catalyze the reaction. However, the resulting hemiketal is prone to hydrolysis under acidic conditions, so neutralization is required at the end of the reaction to stabilize the cross-linked product [27]. In addition, the hydroxyl groups of hyaluronic acid can also be activated by cyanogen bromide and reacted with amine compounds in an aqueous phase to form carbamates [28].
3. 3 Deacetylation and amination
The free amino group formed by deacetylation of the acetyl group on hyaluronic acid can also be used as an active site for modification reactions. It can react with activated carboxylic acids to form amide compounds, or even undergo self-crosslinking with its own carboxyl group to form a gel. However, deacetylation, even under mild conditions, can cause degradation of hyaluronic acid [29], so this method is generally not used for hyaluronic acid modification.
3. 4 Complex modification
Hyaluronic acid can also be used in combination with other materials to take advantage of their respective advantages and compensate for their deficiencies. For example, hyaluronic acid and chitosan can be combined to form nanoparticles through electrostatic interaction, which can be used to load papain and form new surfactants[30]; hyaluronic acid and gelatin can be combined through emulsification-coagulation, and smooth, wrinkled and porous microspheres can be obtained by different post-treatment methods [31]; the combination of hyaluronic acid and hydroxypropyl methyl cellulose can improve the resistance of the gel to enzymatic hydrolysis [32, 33]; the combination of hyaluronic acid and collagen will give it better mechanical properties.
3. 5 Metal complexes
Hyaluronic acid is rich in O and N atoms, and can form coordination bonds with a variety of metal ions, such as Fe3+, Zn2+, Cu2+, Ni2+, etc. Coordination changes the structure of hyaluronic acid in solution and gives it more biological functions[34]. For example, Curiosin gel from Gedeon Richter is a complex of hyaluronic acid and Zn2+, which changes the structure of hyaluronic acid from a random coil to a spherical structure through coordination, reducing the thickness of the bound water molecule layer and making the bond more stable. Clinical trials have shown that this gel can effectively promote wound healing and prevent wound infection.
4 Biomedical applications of hyaluronic acid and its derivatives
Hyaluronic acid's unique properties make it suitable for a wide range of biomedical applications. Balazs [35] divides the clinical applications of hyaluronic acid and its derivatives into five categories.
(1) Viscosurgery: protecting fragile tissues and providing space for surgical operations, such as ophthalmic surgery;
(2) viscoaugmentation: filling and expanding tissue spaces, such as skin, sphincters, vocal cords and pharyngeal tissue;
(3) viscoseparation: separating damaged connective tissue surfaces caused by surgery or trauma to prevent adhesion and excessive scarring;
(4) viscous supplementation (viscosupplimentation): replacing or supplementing tissue fluid, such as replacing the lubricating fluid in arthritis, to relieve pain;
(5) viscous protection (viscoprotection): protecting healthy or damaged tissue surfaces from drying out or the effects of harmful environments, and promoting tissue surface healing.
4.1 Ophthalmology
Hyaluronic acid is a major component of the eye's vitreous humor and is mainly used in ophthalmic surgery to replace the vitreous humor lost during operations such as cataract surgery or intraocular lens implantation. On the other hand, hyaluronic acid is also used as a viscoelastic protective agent in ophthalmic surgery to protect the corneal epithelium, buffer mechanical shocks, maintain the appropriate depth and shape of the anterior chamber of the eye, protect intraocular tissues, prevent vitreous body prolapse, and facilitate surgical operations [36]. Hyaluronic acid is also the main ingredient in eye drops for the treatment of dry eye syndrome. It can effectively prolong the tear film rupture time, reduce the number of blinks in patients with dry eye syndrome, and relieve the symptoms of dryness, irritation, itchiness, and pain.
4. 2 Skin fillers
Hyaluronic acid is a natural moisturizer that is widely found in skin tissue, and its concentration can reach 2. 5g/L. As we age, the amount of hyaluronic acid in the skin gradually decreases, leading to dehydration of the dermis, deepening of wrinkles, and loss of elasticity. Hyaluronic acid is widely used as a skin filler to treat facial aging due to its high viscoelasticity, plasticity, biodegradability, good biocompatibility, and lack of species specificity. According to statistics from the International Society of Aesthetic Plastic Surgery (ISAPS), the number of cases in which hyaluronic acid fillers are used ranks second among minimally invasive cosmetic treatments, after botulinum toxin. However, the natural hyaluronic acid in the human body has a very short maintenance cycle and cannot guarantee the long-term effect of filling and modification. Therefore, physical or chemical cross-linking protection methods are generally used to increase the resistance of hyaluronic acid to enzymatic hydrolysis and prolong its retention time in the body.

4. 3 Anti-adhesion and wound healing
Postoperative tissue adhesion is a major problem in surgical procedures, which can lead to serious long-term clinical complications, affecting the results of surgical procedures and causing pain and inconvenience to patients. A large number of studies have shown that hyaluronic acid plays an important role in preventing adhesion and promoting wound healing. The mechanism of hyaluronic acid in preventing tissue adhesion mainly includes: (1) separating tissues through physical shielding, which can also shield inflammatory mediators and bacteria, thus playing a protective role; (2) promoting the dissolution of blood fibrin, while stimulating the expression of CD44 receptors to promote the proliferation of mesenchymal cells; (3) enhancing the function and activity of macrophages, regulating collagen synthesis, reducing the deposition of blood fibrin, promoting wound healing and reducing scar formation; (4) forming a protective film on the tissue surface to reduce mechanical damage and provide lubrication and moisture; (5) absorbing and expanding to compress bleeding points and suppress bleeding[38] .
Epidermal growth factor (EGF), fibroblast growth factor (bFGF), etc. are now widely used in skin wound repair, but these products are generally in the form of freeze-dried powder, which needs to be stored in the refrigerator before use, and has a very short half-life, so it needs to be applied repeatedly every day. Yamamoto et al. [39] reported a dual-layer wound dressing formed by high-molecular-weight and low-molecular-weight hyaluronic acid, where the high-molecular-weight cross-linked hyaluronic acid forms the upper layer of the supplement and the low-molecular-weight hyaluronic acid, arginine, vitamin C derivatives and EGF form the lower layer. Experimental results show that this wound supplement can maintain the activity of EGF and promote the release of vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF).
4. 4 Arthritis
Hyaluronic acid is the main component of articular cartilage and synovial fluid. In normal, healthy joints, movement can be carried out almost friction-free and pain-free. However, when joint diseases such as osteoarthritis or rheumatoid arthritis occur, the concentration of hyaluronic acid in the synovial fluid decreases significantly, the molecular weight decreases significantly, and the cartilage is also degraded and destroyed, causing the joint movement to become stiff and pain due to bone-on-bone friction. Injecting exogenous high molecular weight hyaluronic acid into the joint restores the synovial fluid to a normal state and promotes the gradual natural repair of cartilage. At the same time, the injected hyaluronic acid also improves the biological environment of the joint cavity, promotes the synthesis of endogenous hyaluronic acid, and improves joint function. However, because the half-life of hyaluronic acid in the body is short, repeated and frequent injections are required for the treatment of joint lesions, which increases the patient's suffering. Recently, Jordan et al. [40] reported a new type of gel made from a mixture of hyaluronic acid and chitosan. The addition of chitosan not only improves the anti-degradation ability of hyaluronic acid, but also improves its therapeutic effect. This research provides a new direction for improving the viscous hyaluronic acid supplements for the treatment of joint disease.
In recent years, there has been some debate about whether this viscoelastic supplement therapy is effective in treating arthritis. The second edition of the “Evidence-Based Guidelines for the Treatment of Knee Osteoarthritis” issued by the American Academy of Orthopaedic Surgeons in 2013 clearly states that hyaluronic acid is not recommended for the treatment of symptomatic knee osteoarthritis. They believe that although many studies have shown that the effect of high molecular weight hyaluronic acid on the treatment of osteoarthritis is statistically different compared to the control, this difference does not meet the minimum clinically important difference (MCII) standard and therefore does not have a clinically significant difference.
4. 5 Drug carrier
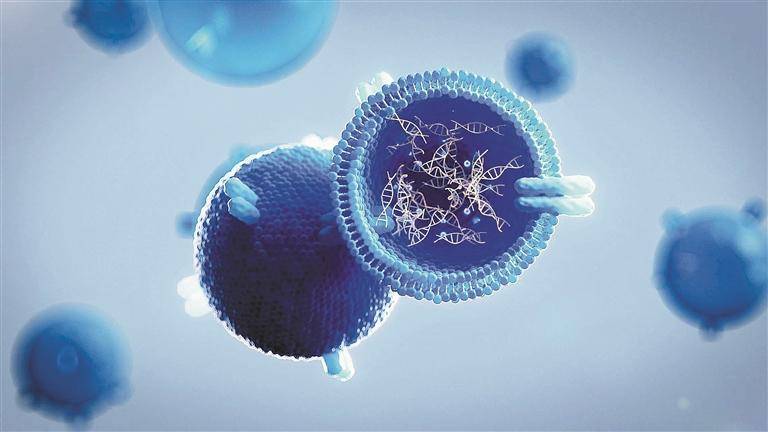
Hyaluronic acid has the potential to be used as a drug carrier due to its good biocompatibility, high hydrophilicity, high viscoelasticity, degradability and specific binding to cell surface receptors (such as CD44 and RHAMM). On the other hand, from the chemical structure of hyaluronic acid, it has multiple reaction sites, including carboxyl groups, hydroxyl groups and acetylated amino groups, which can be used to construct drug precursors and carriers using a variety of chemical modification methods. At present, hyaluronic acid and its derivatives have been used to construct drug delivery systems for a variety of drugs, including anti-inflammatory drugs, anti-tumor drugs, protein peptide drugs and gene drugs, which can significantly prolong the blood circulation residence time of drugs, increase cellular uptake, improve bioavailability, reduce the amount of drug administered, and reduce adverse reactions [8, 41]. Zhong et al. [42] reported a reduction-sensitive, reversibly cross-linked hyaluronic acid nanoparticle composed of a hyaluronic acid-lysine-lipoic acid (HA-Lys-LA) covalent bond, which is cross-linked by a disulfide bond under the catalysis of 1, 4-dithio-D,L-threitol (DTT) catalysis, the drug doxorubicin (DOX) is cross-linked by disulfide bonds to improve the residence time of the drug under physiological conditions.
This nanocarrier specifically binds to the CD44 receptor overexpressed on the surface of MCF-7 human breast cancer cells resistant to DOX through hyaluronic acid located on the surface, thereby increasing the cellular uptake of the drug. The nanocarrier then swells and releases the drug by catalysing the breakage of the disulfide bond by glutathione, which is overexpressed in tumor cells, effectively inhibiting tumor growth (as shown in Figure 6). Park et al. [43] also reported the use of a similar drug carrier for siRNA transfection. They designed and synthesized a hyaluronic acid-poly(dimethylaminoethyl methacrylate) (HPD) grafted polymer as a siRNA delivery vehicle, and cross-linked it via a disulfide bond. In vitro experiments have shown that the cross-linked siRNA complex (C-siRNA-HPD) is more stable and can be more effectively taken up by melanoma cells overexpressing CD44, thereby improving siRNA transfection efficiency. In vivo experiments have shown that after systemic administration in mice, C-siRNA-HPD selectively accumulates in tumors, demonstrating its tumor targeting properties.
4. 6 Tissue engineering
Tissue engineering is a new interdisciplinary subject that emerged in the 1980s and has become a research hotspot in tissue and organ regeneration medicine in recent years. Hyaluronic acid is an important component of many tissues in the human body and is a major component of the extracellular matrix. It affects cell proliferation, migration and differentiation, and promotes wound healing, making it an ideal raw material for tissue engineering. However, the weak mechanical properties, high swelling properties, smooth surface structure and lack of resistance to enzymatic hydrolysis of hyaluronic acid gels also limit their application in tissue engineering. Therefore, in order to improve the possibility of using them as scaffolds for tissue engineering, necessary chemical modifications are required to compensate for their deficiencies. One good method is to select other biomaterials for compounding, which can combine the advantages of multiple materials to complement each other's shortcomings. For example, sodium alginate and hyaluronic acid can be cross-linked to form a porous composite gel. By adjusting the concentration of the polymer and the ratio of the two polysaccharides, the expansion rate, porosity and resistance to enzymatic hydrolysis of the composite gel can be controlled, so that it can provide a good biological environment for cell attachment and proliferation [44].
4. 7 Biomimetics
High-throughput screening of drugs is generally done through 2D in vitro cytological evaluation, but this method differs greatly from the actual results in vivo. Using 3D scaffolds to simulate the cell microenvironment is more in line with actual conditions. Hyaluronic acid is an important component of the extracellular matrix, and using it to construct a 3D culture medium will be more suitable for mimicking the in vivo growth environment of cells. Hyaluronic acid itself is negatively charged, which hinders cell adhesion, so it needs to be combined with other biomaterials to promote cell adhesion. Zhang et al. [45] used hyaluronic acid and chitosan to construct a 3D porous scaffold material for mimicking the extracellular matrix microenvironment of U-118MG human malignant glioma cells as a 3D culture medium for high-throughput screening of anti-tumor drugs. Compared with 2D culture medium, hyaluronic acid-chitosan scaffold culture medium can promote the formation of tumor spheroids and upregulate the expression of CD44, nestin, Musashi-1, GFAP and HIF-1α proteins.
5 Conclusion
Hyaluronic acid has a history of more than 60 years since it was first used in human medicine in the late 1950s. Due to its special rheological properties and physiological functions, hyaluronic acid is widely used in the biomedical field. So far, the research on the design of new hyaluronic acid derivatives has made great progress, and more and more hyaluronic acid products have been developed to fill the gaps in biomedical applications. This paper reviews the structural properties, synthetic modification and biomedical applications of hyaluronic acid. However, there are still many unanswered questions about the physiological functions of hyaluronic acid. At present, commercially available hyaluronic acid biomaterials still have certain defects that require further improvement to promote the wider application of hyaluronic acid in the biomedical field.
Reference
[ 1 ]Meyer K , Palmer JW. J Biol Chem , 1934 , 107 : 629~634.
[ 2 ]Balazs EA , Laurent TC , Jeanloz RW. Biochem J , 1986 , 235 : 903~903.
[ 3 ]Weigel PH , Hascall VC , Tammi M. J Biol Chem , 1997 , 272 : 13997~14000.
[ 4 ]Kogan G , Soltes L , Stern R , Gemeiner P. Biotechnol Lett , 2007 , 29 : 17~25.
[ 5 ]Csoka AB , Frost GI , Stern R. Matrix Biol , 2001 , 20 : 499~508.
[ 6 ]De Boulle K , Glogau R , Kono T , Nathan M , Tezel A , Roca-Martinez J-X , Paliwal S , Stroumpoulis D. Dermatol Surg , 2013 , 39 : 1758~1766.
[ 7 ]Termeer C , Sleeman JP , Simon JC. Trends Immunol , 2003 , 24 : 112~114.
[ 8 ]Schante CE , Zuber G , Herlin C , Vandamme TF. Carbohydr Polym , 2011 , 85 : 469~489.
[ 9 ]Jiang D , Liang J , Fan J , Yu S , Chen S , Luo Y , Prestwich GD , Mascarenhas MM , Garg HG , Quinn DA , Homer RJ , Goldstein DR , Bucala R , Lee PJ , Medzhitov R , Noble PW. Nat Med , 2005 , 11 : 1173~1179.
[10] Ling Peixue, He Yanli, Zhang Qing. Food and Drugs, 2005 , 7 : 1~3.
[11] Jin Yan, Li Dawei, Zhu Meihua , Chen Jianying. Food and Drugs, 2014 , 16 : 373~376.
[12] Cui Yuan, Duan Qian, Li Yanhui. Journal of Changchun University of Science and Technology (Natural Science Edition), 2011 , 34 : 101~106.
[13]Brown TJ , Laurent UBG , Fraser JRE. Exp Physiol , 1991 , 76 : 125~134.
[14]Danishefsky I , Siskovic E. Carbohydr Res , 1971 , 16 : 199~205.
[15]Magnani A , Rappuoli R , Lamponi S , Barbucci R. Polym Adv Technol , 2000 , 11 : 488~495.
[16]Bergman K , Elvingson C , Hilborn J , Svensk G , Bowden T. Biomacromolecules , 2007 , 8 : 2190~2195.
[17]Bellini D , Topai A. WO2000001733A1. 2000.
[18]Bulpitt P , Aeschlimann D. J Biomed Mater Res , 1999 , 47 : 152~169.
[19]Pouyani T , Prestwich GD. Bioconjugate Chem , 1994 , 5 : 339~347.
[20]Pelletier S , Hubert P , Lapicque F , Payan E , Dellacherie E. Carbohydr Polym , 2000 , 43 : 343~349.
[21]Bencherif SA , Srinivasan A , Horkay F , Hollinger JO , Matyjaszewski K , Washburn NR. Biomaterials , 2008 , 29 : 1739~1749.
[22]Laurent TC , Hellsing K , Gelotte B. Acta Chemica Scandinavica , 1964 , 18 : 274~275.
[23] Jun Jian, Ruizhi Li. cn 102321258b. 2012.
[24]De Belder AN , Malson T. US4886787A. 1985.
[25]Tomihata K , Ikada Y. Biomaterials , 1997 , 18 : 189~195.
[26]Coradini D , Pellizzaro C , Miglierini G , Daidone MG , Perbellini A. Int J Cancer , 1999 , 81 : 411~416.
[27]Collins MN , Birkinshaw C. J Appl Polym Sci , 2007 , 104 : 3183~3191.
[28]Mlcochova P , Bystricky S , Steiner B , Machova E , Koos M , Velebny V , Krcmar M. Biopolymers , 2006 , 82 : 74~79
[29]Crescenzi V , Francescangeli A , Segre AL , Capitani D , Mannina L , Renier D , Bellini D. Macromol Biosci , 2002 , 2 : 272~279.
[30]Zhao D , Wei W , Zhu Y , Sun J , Hu Q , Liu X. Macromol Biosci , 2015 , 15 : 558~567.
[31]Zhou Z , He S , Huang T , Peng C , Zhou H , Liu Q , Zeng W , Liu L , Huang H , Xiang L , Yan H . Polym Bull , 2015 , 72 : 713 ~723.
[32] Jian Jun, Li Ruizhi. CN 102492180B. 2014.
[33] Jian Jun, Li Ruizhi. CN 102911380A. 2013.
[34] Jin Yan, Ling Peixue, Zhang Tianmin. Chinese Journal of Biochemical Drugs, 2008, 29427-429.
[35] Garg HG, Hales CA. Chemistry and Biology of Hyaluronan, UK: Elsevier, 2004, 415-455.
[36] Zhang Lei, Wu Di, Sun Wei, Sun Junde. Journal of Microbiology, 2006, 26: 100-103.
[37] Pang Suqiu, Zhou Jinsheng, Chen Qiuxia. Strait Pharmacy, 2003, 15: 252.
[38] Ling Peixue, Guan Huashi. Chinese Journal of Pharmacy, 2005, 40: 1527-1530.
[39]Yamamoto A , Shimizu N , Kuroyanagi Y. J Artif Organs , 2013 , 16 : 489~494.
[40]Kaderli S , Boulocher C , Pillet E , Watrelot-Virieux D , Rougemont AL , Roger T , Viguier E , Gurny R , Scapozza L , Jordan O. Int J Pharm , 2015 , 483 : 158~168.
[41] Zhang Wei, Yan Cui'e. Chemical Progress, 2006, 18: 1684~1690.
[42] Zhong Y, Zhang J, Cheng R, Deng C, Meng F, Xie F, Zhong Z. J Controlled Release, 2015, 205: 144–154.
[43] Yoon HY, Kim HR, Saravanakumar G, Heo R, Chae SY, Um W, Kim K, Kwon I C, Lee JY, Lee DS, Park JC, Park JH. J Controlled Release , 2013 , 172 : 653~661.
[44]Chen Y H, Li J, Hao Y B, Qi J X, Dong N G, Wu C L, Wang Q. J Appl Polym Sci, 2015, 132: 41898.
[45] Florczyk SJ, Wang K, Jana S, Wood DL, Sytsma SK, Sham JG, Klevit FM, Zhang M. Biomaterials, 2013, 34: 10143–10150.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese