Lutein What Is It Good For?
Lutein is a natural xanthophyll containing two different violone rings. It is a non-vitamin A active carotenoid that is widely found in vegetables, fruits, flowers, etc. It is an important pigment in the macular region of the human eye's retina[1]. Lutein contains a unique dihydroxy structure with a violone ring, which can be used as a strong antioxidant to quench singlet oxygen and as a blue light filter. It has biological effects such as anti-oxidation, anti-cancer, protecting the retina and preventing cardiovascular disease. However, lutein has unstable physicochemical properties, and there are problems such as poor water solubility, instability and low bioavailability during the preparation process. Lutein can only be absorbed in the body by binding to lipids, and this characteristic greatly restricts the exertion of its pharmacological effects.
1. The physical and chemical properties of lutein
Natural lutein is mainly composed of two different violone rings linked by a long chain containing an 18-carbon atom conjugated double bond. It has three chiral centers and eight stereoisomers. It has a rhombic bright yellow crystal with a metallic luster and can absorb blue and purple light well. Natural lutein is mainly in the all-trans configuration, while in human serum and plasma lutein is mainly in the 3R, 3, R, 6, R configuration. Lutein is insoluble in water and propylene glycol, slightly soluble in oil and hexane, soluble in acetone, dichloromethane and ethanol, and easily soluble in ethyl acetate, tetrahydrofuran, chloroform, etc. [2]. Its stability in solvents is anhydrous ethanol > ethyl acetate > tetrahydrofuran > toluene. Lutein is unstable and is mainly affected by factors such as oxygen, light, heat, metal ions, and pH. For example, heat treatment can cause the isomerization of lutein to produce 9-cis and 13-cis lutein [3]. Therefore, when stored, lutein crystals or materials containing lutein should be sealed in airtight containers or vacuum-sealed and filled with inert gas, protected from light, and stored at low temperatures.
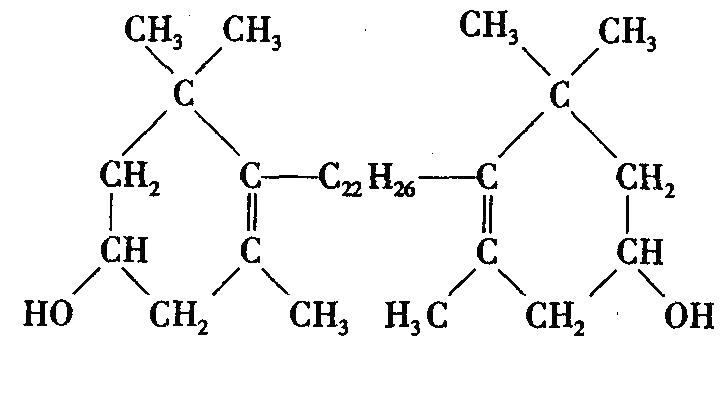
In nature, lutein mainly exists in the form of free lutein and lutein esters [4]. The bioavailability of free lutein is low, only 2% to 9.4% [5]. The bioavailability of lutein is effectively improved after it is prepared into lutein products (oil suspension or microcapsules) [6-8]. Lutein esters are more stable than the free form and have a 1.6-fold higher bioavailability in vivo than free lutein [9-10]. Therefore, lutein has problems such as poor water solubility, instability and low bioavailability during preparation.
2 In vivo process of lutein
Lutein is ingested in micelles with lipids. It is released from the stomach by various enzymes, absorbed into intestinal cells, transported via the lymphatic or portal vein with the chylomicrons into the blood circulation and then into the liver. In the liver, it is converted and released into the blood circulation together with low-density lipoproteins (LDL). Some of the lutein in the blood is bound to other corresponding carriers and enters the retina and other tissues.
2.1 Absorption and distribution
Lutein synthase is not found in the human body, so lutein can only be obtained through dietary intake. Lutein in food is combined with lipids to form mixed lipid micelles, which are absorbed by the body through cholesterol transport or passive diffusion. Lu Ping et al. [11] showed that lutein microcapsules were absorbed in all intestinal segments of rats, but their absorption rate constants were different, in descending order: ileum > jejunum > duodenum > colon [11]. After entering the body, lutein is stored in fat and is mainly distributed in the liver, blood and retina. Under normal dietary conditions, the concentrations of lutein in human plasma, serum, liver, kidney and lung are 0. 14 to 0.61, 0. 10 to 1.23, 0. 10 to 3.00, 0.037 to 2.10 and 0. 10 to 2.30 μmol/L, respectively [12]. Lutein is found in all ocular tissues, with the highest concentration around the macula of the retina, up to 0.1–1 mmol/L [13].
2.2 Transport
The B-type scavenger receptors involved in the transport of lutein in the body are mainly SR-BI and CD36. SR-BI is a high-density lipoprotein (HDL) receptor, and lutein can be transported to retinal pigment epithelial cells through the SR-BI mechanism [14]. CD36, as a multi-ligand receptor, promotes the uptake and modification of specific lipid molecules such as long-chain free fatty acids on LDL [15-16]. CD36 binds to the outer segment of rod photoreceptors in association with rhodopsin and phospholipids, thereby mediating the metabolism of the outer segment of the photoreceptor; the CD36 receptor can also bind to the interphotoreceptor retinoid-binding protein, which transports lutein to retinal cells [17].
2.3 Metabolism
BCO1 and BCO2 are carotenoid cleavage enzymes found in animals, including the retina and retinal pigment epithelial cells (RPE) [18]. There are two metabolic pathways for lutein in the body. One is the symmetric decomposition of the double bond in the molecule by the enzyme β-carotene-15,15, -oxygenase (BCMO1), with the main metabolites being vitamin A and its derivatives. The other is the asymmetric decomposition of the double bond in the alkenyl group by the enzyme β-carotene-9,10, -double oxygenase (BCDO2) [19]. BCDO2 is mainly responsible for the production of macular pigment in retinal epithelial cells.
3 Lutein's antioxidant activity
Reactive oxygen species (ROS) can react with DNA, proteins and lipids, impairing their physiological functions and leading to the development of chronic diseases such as atherosclerosis, cancer and macular degeneration. Lutein exerts its biological effects by forming a cationic radical with a conjugated polyene chain losing an electron, thereby reducing oxygen free radicals and inhibiting the activity of ROS, preventing damage to normal cells by ROS[20-21] . Lutein is known as a natural antioxidant that can quench singlet oxygen, capture oxygen radicals and prevent damage to biological membranes by free radicals.
Singlet oxygen is a type of excited ROS that often acts as an inducer of chain oxidation reactions. Lutein can inactivate singlet oxygen through physical or chemical quenching, thereby protecting the body from harm and enhancing its immune function. Hydroxyl radicals are the most active type of ROS, which can initiate chain reactions of peroxidation of unsaturated fatty acid lipids, producing a series of free radicals such as lipid radicals, lipid oxygen radicals, lipid peroxyl radicals and lipid peroxides. Lutein can scavenge free radicals, especially hydroxyl radicals. Lutein is a chain-breaking antioxidant that can bind to lipids and effectively inhibit lipid oxidation, thus protecting cells and organs from the damage caused by free radicals in the body.
4 Biological effects of lutein
4.1 Protective effect on the retina
The retina is rich in blood vessels and has a high oxygen concentration. It also contains photosensitive compounds and easily oxidized substrates. Under high-energy light conditions, oxygen free radicals are easily produced, leading to lipid peroxidation in retinal cells, causing the epitopes of intracellular proteins to become denatured and DNA damage, ultimately leading to retinal cell apoptosis. The protective mechanism of lutein in the retina is to reduce ROS damage and filter blue-violet light[22]. With age, the RPE gradually accumulates lipofuscin [23-24], and lipofuscin photosensitization can generate a large number of reactive oxygen species. Lutein can effectively prevent the oxidative reaction initiated by the retinal photosensitizer A2-PE and reduce RPE phototoxicity by reducing ROS levels and inhibiting oxidative stress [25]. Lutein also reduces the chance of mitochondrial apoptosis by inhibiting mitochondrial superoxide production in retinal vascular endothelial cells, preventing endothelial cell apoptosis and reversing degenerative changes in capillaries [26].
Macular pigment is concentrated in the layer of Henle's fiber cells, which are made up of many photoreceptor nerve axons [27]. Light must pass through macular pigment to reach the photoreceptors. Lutein is similar to a filter in the inner layer of the retina, absorbing blue light before it reaches the photoreceptors and the RPE and the underlying choroidal vascular layer, reducing the light energy and thus reducing the photo-oxidation of light-sensitive substances in the RPE. In a study of the effects and mechanisms of lutein on diabetic retinopathy, Wang Liyuan et al. [28] found that lutein can relieve the oxidative stress caused by diabetic retinopathy, protect retinal vascular endothelial cells, and reduce retinal damage.
4.2 Preventive effect on cardiovascular disease
The accumulation of cholesterol and LDL in the human body causes the arteries to close and thicken, and the walls of the blood vessels lose their elasticity, which in turn leads to cardiovascular diseases such as atherosclerosis. Lutein can inhibit the lipid peroxidation of LDL through its antioxidant effect, thereby delaying the formation of arterial plaques and preventing the occurrence of atherosclerosis and other arterial cardiovascular diseases. The change in the thickness of the intima of the carotid artery main channel is related to the lutein content in the blood. Under certain conditions and within a certain range, the thickness of the blood vessel wall is negatively correlated with the lutein content. Lutein can also inhibit the oxidative modification of LDL and prevent the proliferation of smooth muscle cells [29].
Riccioni G et al. [30] found that dietary supplementation with lutein can improve the inflammatory response and oxidative stress of endothelial cells, delay the formation of atherosclerosis, and thus reduce the risk of coronary heart disease.
4.3 Anti-cancer effect
At present, the mechanism of lutein's anticancer effect is not clear, but it is mainly believed to have the following points: (1) Lutein can quench singlet oxygen and prevent lipid peroxidation, thereby inhibiting tumor growth. Sun Guogui et al. [31] found that compared with normal liver cells, the ROS level of liver cancer cells is significantly higher, and excessive ROS can cause damage to nuclear DNA, mutations in mitochondrial DNA, and peroxidation of proteins and lipids.
In a study on the inhibitory effect and mechanism of lutein on human liver cancer cells HepG2, Wang Ruozhong et al. [32] found that lutein, which has antioxidant properties, can effectively reduce the ROS level in HepG2 cells, thereby interrupting its important role in tumorigenesis and development. This may be one of the reasons why lutein can inhibit HepG2 cell proliferation. (2) Lutein can enhance the humoral and cellular immunity of cells in the body and inhibit the growth of cancer cells in the body. The presence of a polar gene at the end of the lutein structure may enhance the growth of antigen-presenting lymphocytes and affect the functional expression of cell surface molecules [33], thereby improving self-immunity. (3) It may also indirectly regulate immunity through the synergistic effect of other organs in the human body, thereby preventing cancer. Gunasekera RS et al. [34] studied the inhibitory effect of lycopene and lutein on rat prostate cancer cells, and the results showed that both lycopene and lutein can inhibit the growth of malignant tumor cells AT3.
5 Research on the main dosage forms of lutein
When lutein powder is used in clinical or food applications, it mainly faces problems such as poor water solubility, instability and low bioavailability. Isomerization and oxidation reactions inevitably occur during the preparation, storage and processing of lutein [35]. The dosage form needs to be changed by methods such as physical encapsulation and chemical modification (such as esterification) to improve its bioavailability. The main dosage forms currently on the market are described below.
5.1 Oil suspension
Lutein crystals are micronized to the micron level, vegetable oil and antioxidants are added, and the mixture is stirred properly to prepare an oil suspension containing about 20% lutein. The key step is to prevent lutein from thermal degradation during micronization. Ye Wenkun et al. [36] used the emulsification method to prepare a lutein oil suspension, and determined that the optimal process was as follows: a mixture of 4% lecithin and mono-stearin glycerides (mass ratio 1:1) was used as an emulsifier, and the emulsification temperature was 65 °C. The antioxidant (vitamin E) and caprylic capric triglyceride were stirred well, and then a quantitative amount of lutein powder was added. emulsified at 16,000 rpm for 30 minutes to obtain a yellow, viscous liquid containing 20% lutein.
5.2 Water-dispersible dry powder
A water-dispersible dry powder is a solid dispersion of lutein in a suitable carrier in the form of a microcrystal, amorphous form, colloidal dispersion or molecular dispersion, which can be dispersed in cold water. Li Sen et al. [37] used polyethylene glycol 6000 and poloxamer 188 as carriers to prepare lutein solid dispersions by the solvent method. The results showed that lutein exists in the form of a low melting mixture in the carrier. The use of water-soluble carriers to prepare lutein solid dispersions not only improves the solubility and dissolution rate of the drug, but also increases the wettability of the drug and maintains a highly dispersed state of the drug. Zhang Tianye et al. [38] prepared a temperature-sensitive in situ gel from the lutein solid dispersion by the cold melting method, which not only solved the problem of poor water solubility of lutein, but also significantly increased the residence time of the drug in the conjunctival sac, achieving a sustained release effect.
5.3 Microcapsules
Microcapsules are tiny capsules that encapsulate drugs and release them under certain conditions. The typical particle size is between 0.7 and 5 µm. The particle size of traditional microencapsulated agents is too large, and the surface effect is low. Therefore, Chinese and foreign scholars have explored the use of nanoparticle and nanomicrocapsule composite systems to encapsulate lutein [39-40]. The advantages of lutein microcapsules are that they preserve the biological activity, control the release, and improve the instability and water solubility of lutein. Wang et al. [41] used octenyl succinate-modified starch and sucrose as wall materials, and trans-lutein crystals as core materials to prepare lutein microcapsules by emulsifying, homogenizing, and spray drying. On this basis, secondary embedding was carried out using condensation spray drying to make the product more stable. This process is suitable for industrial production.

5.4 Liposome
Liposomes are a new type of microcapsule dosage form. They are used as nano-carriers to deliver functional factors. They have a small particle size and a large surface area. They are non-toxic, non-immunogenic and biodegradable. They can increase the dissolution of drugs and promote their absorption in the human body [42-43]. After drugs encapsulated in liposomes are made into eye drops, they can increase corneal permeability, slow drug release and reduce drug toxicity. Among the carotenoids, liposomes have the strongest ability to encapsulate lutein. They can be used to prepare lutein liposomes with high loading capacity, small particle size and good dispersibility. In addition, there is a synergistic protective effect between liposomes and lutein, which can effectively inhibit the instability of liposomes. It is worth noting that high concentrations of carotenoids can aggregate when loaded into liposomes, which may lead to changes in the fluidity and permeability of the lipid membrane, thereby promoting oxidation [44].
Tan Chen [40] prepared lutein liposomes by thin-film sonication method, using egg yolk lecithin and cholesterol as membrane materials, at 50 °C and with a drug loading of 1.25%, the mixture was rotated evaporated for 60 minutes, and then ultrasonicated in an ice bath for 2 minutes. The encapsulation rate of the obtained lutein liposomes was over 90%, the particle size distribution was uniform, the particle size was about 80 nm, and the in vitro antioxidant properties were good. The results show that liposomes, as a carrier, can effectively solve the instability of lutein, effectively inhibit the aggregation and fusion of liposomes and the leakage of core materials, reduce the fluidity of the bilayer of liposomes, and improve the physical stability of liposomes. Xia F et al. [45] prepared lutein liposome precursors based on the supercritical fluid dispersion method. This process is environmentally friendly and has great potential for industrial application in the preparation of liposome precursors.
6 Current status of lutein applications
Lutein is one of the main active ingredients in health products for “relieving visual fatigue” approved for marketing by the China Food and Drug Administration. Lutein ester was approved as a new resource food by the former Ministry of Health in Announcement No. 12 of 2008. In 2008, Lutein Ester Tablets produced by North China Pharmaceutical Co., Ltd. contained 3.2 mg of lutein per tablet. In 2013, Shanghai Grape King Enterprise Co., Ltd. launched a vision fatigue relief drink containing 3.2 mg of lutein per 100 mL of drink. The company By-Health Co., Ltd. has launched a soft capsule to relieve eye fatigue, each capsule containing 5 mg lutein.
Lutein is still being studied as a medicine, and there have been no reports of lutein prescription drugs at home or abroad. However, relevant research has shown that lutein has a certain effect on ophthalmic diseases. For example, Hu Bojie et al. [46] studied the clinical application of lutein and zeaxanthin in diabetic retinopathy. For patients with simple diabetic retinopathy, there is a positive correlation within a certain range between visual acuity and the content of lutein and zeaxanthin in the serum. Xia Liying et al. [47] conducted a clinical study on lutein for the treatment of age-related macular degeneration, and the results showed that the serum concentrations of lutein and zeaxanthin were positively correlated with the concentration of macular pigment in the retina.
7 Conclusion
In summary, lutein is a natural antioxidant with excellent free radical scavenging and light shielding abilities. It has a wide range of biological effects, including anti-tumor and immunomodulatory effects, as well as anti-cardiovascular disease effects. It can also effectively prevent and treat diseases of the macular region of the eye (such as cataracts, age-related macular degeneration, diabetic retinopathy, etc.), and has great potential in the development of drugs for the treatment of diseases of the macular region of the eye. However, there are still many problems with the development and utilization of lutein, such as the large particle size of traditional preparations, the influence of gastrointestinal action and the low bioavailability, which need to be studied in depth. Nanoparticle preparation and nano-liposome preparations that mimic cell membrane structures can be considered in the preparation process to maximize bioavailability, reduce the dose and toxicity of the drug, and also protect lutein from destruction by gastrointestinal enzymes. Further research could be carried out on liposomal lutein eye drops in the future.
References
[ 1 ] Wang Yanbo, Shi Yan, Tang Huian. Research progress on the extraction, efficacy and application of lutein [J]. China Brewing, 2011 (7): 1-4.
[2] Zhu Haixia, Zheng Jianxian. Structure, distribution, physicochemical properties and physiological functions of lutein [J]. China Food Additives, 2005 (5): 48-55.
[3] Aparicio-Ruiz R,Mínguez-Mosquera MI,Gandul-Rojas B. Thermal degradation kinetics of lutein,β-carotene and β- cryptoxanthin in virgin olive oils[J]. J Food Compost Anal,2011,24(6):811-820.[ 4 ] Blesso CN,Andersen CJ,Bolling BW,et al. Egg intake improves carotenoid status by increasing plasma HDL cho- lesterol in adults with metabolic syndrome[J]. Food Funct,2013,4(2):213-221.
[ 5 ] Lienau A,Glaser T,Tang G,et al. Bioavailability of lutein in humans from intrinsically labeled vegetables deter- mined by LC- APCI- MS[J]. J Nutr Biochem,2003,14 (11):663-670.
[ 6 ] Cha KH,Lee JY,Song DG,et al. Effect of microfluidization on in vitro micellization and intestinal cell uptake of lutein from Chlorella vulgaris[J]. JAgric Food Chem, 2011,59(16):8670-8674.
[ 7 ] Shanmugam S,Baskaran R,Balakrishnan P,et al. Solid selfnanoemulsifying drug delivery system(S-SNEDDS) containing phosphatidylcholine for enhanced bioavailabili- ty of highly lipophilic bioactive carotenoid lutein[J]. Eur J Pharm Biopharm,2011,79(2):250-257.
[ 8 ] Kalariya NM,Ramana KV,vanKuijk FJGM. Focus on molecules:lutein[J]. Exp Eye Res,2011,40(1):107-108.
[9] Wu Yang, Li Shuguo. Properties, functions and applications of natural edible lutein in food processing [J]. Food Science and Technology and Economics, 2015, 40 (1): 59-62.
[10] Granado- Lorencio F,Herrero- Barbudo C,Olmedilla- Alonso B,et al. Lutein bioavailability from lutein ester- fortified fermented milk:in vivo and in vitro study[J]. J Nutr Biochem,2010,21(2):133-139.
[11] Lu Ping, Wang Xinchun, Chen Wen, et al. Study of the in vivo intestinal absorption of lutein microcapsules in rats by the unidirectional perfusion method [J]. Chinese Journal of Experimental Pharmacology, 2011, 17 (18): 133-136.
[12] Olmedilla-Alonso B,Beltrán-de-Miguel B,Estévez-San- tiago R,et al. Markers of lutein and zeaxanthin status in two age groups of men and women:dietary intake,se- rum concentrations,lipid profile and macular pigment op- tical density[J]. Nutr J,2014,doi:10. 1186/1475- 2891- 13- 52.
[13] Zhang Wei, Tong Nian-ting, Yin Li-li, et al. Experimental research progress on the role and mechanism of lutein in ophthalmic diseases [J]. Journal of Shanghai Jiaotong University (Medical Sciences), 2012, 32 (2): 231-234.
[14] During A ,Doraiswamy S ,Harrison EH. Xanthophylls are preferentially taken up compared with β-carotene by retinal cells via a SRBI-dependent mechanism[J]. J Lipid Res,2008,49(8):1715-1724.
[15] Silverstein RL,Febbraio M. CD36,a scavenger receptor involved in immunity,metabolism,angiogenesis,and be- havior[J]. Sci Signal,2009,doi:10. 1126/scisignal.272re3.
[16] Silverstein RL,Li W,Park YM,et al. Mechanisms of cell signaling by the scavenger receptor CD36:implications in atherosclerosis and thrombosis[J]. Trans Am Clin Climatol Assoc,2010,121:206-220.
[17] Vachali PP,Besch BM,Gonzalez-Fernandez F,et al. Ca- rotenoids as possible interphotoreceptor retinoid-binding protein(IRBP ) ligands: a surface plasmon resonance
(SPR)based study[J]. Arch Biochem Biophys,2013,539(2):181-186.
[18] Li B,Vachali PP,Gorusupudi A,et al. Inactivity of hu- man β , β-carotene- 9,,10, -dioxygenase(BCO2)under- lies retinal accumulation of the human macular carotenoid pigment[J]. PNAS,2014,111(28):10173-10178.
[19] Wang Y,Chung SJ,McCullough ML,et al. Dietary ca- rotenoids are associated with cardiovascular disease risk biomarkers mediated by serum carotenoid concentrations [J]. JNutr,2014,144(7):1067-1074.
[20] Frank HA,Young AJ,Britton G,et al. The photochemis- try of carotenoids[M]. Springer:Science & Business Me- dia,2006:8-10.
[21] Trevithick- Sutton CC,Foote CS,Collins M,et al. The retinal carotenoids zeaxanthin and lutein scavenge super- oxide and hydroxyl radicals :a chemiluminescence and ESR study[J]. Mol Vis,2006,12:1127-1135.
[22] Kijlstra A,Tian Y,Kelly ER,et al. Lutein:more than just a filter for blue light[J]. Prog Retin Eye Res,2012,31(4): 303-315.
[23] Bhosale P,Serban B,Bernstein PS. Retinal carotenoids can attenuate formation of A2E in the retinal pigment epi- thelium[J]. Arch Biochem Biophys,2009,483(2): 175- 181.
[24] Sparrow JR,Cai B. Blue light-induced apoptosis of A2E- containing RPE:involvement of caspase- 3 and protection by Bcl- 2[J]. Invest Ophthalmol Vis Sci,2001,42(6): 1356-1362.
[25] Bian Q,Gao S,Zhou J,et al. Lutein and zeaxanthin sup- plementation reduces photooxidative damage and modu- lates the expression of inflammation-related genes in reti- nal pigment epithelial cells[J]. Free Radic Biol Med, 2012,53(6):1298-1307.
[26] Sasaki M,Ozawa Y,Kurihara T,et al. Neurodegenera- tive influence of oxidative stress in the retina of a murine model of diabetes[J]. Diabetologia,2010,53(5): 971- 979.
[27] Snodderly DM. Evidence for protection against age-relat- ed macular degeneration by carotenoids and antioxidant vitamins[J]. Am J Clin Nutr,1995,62(Suppl):1448-1461.
[28] Wang Liyuan, Han Yue, Zhang Qiang, et al. The effect and mechanism of lutein on diabetic retinopathy [J]. Foreign Medical Geography, 2015, 36 (4): 311-313.
[29] Zheng Ying, Yang Yuexin, Ma Aiguo. Research progress on the relationship between lutein intake and chronic diseases [J]. Health Research, 2012, 41(1): 144-148.
[30] Riccioni G,Speranza L,Pesce M,et al. Novel phytonu- trient contributors to antioxidant protection against cardio- vascular disease[J]. Nutrition,2012,28(6):605-610.
[31] Sun Guogui, Wang Yadi. Reactive oxygen species and tumors [J]. Carcinogenesis, Mutagenesis and Mutation, 2011, 23(1): 78-80.
[32] Wang Ruozhong, Shen Xinnan, Shi Dongyun, et al. Research on the inhibitory effect and mechanism of lutein on human liver cancer cells HepG2 [J]. Journal of Nutrition, 2012, 34 (4): 332-335.
[33] Li Z. Research progress on the relationship between lutein and immune function [J]. Family Health (Academic Edition), 2015, 9 (5): 286-287.
[34] Gunasekera RS,Sewgobind K,Desai S,et al. Lycopene and lutein inhibit proliferation in rat prostate carcinoma cells[J]. Nutr Cancer,2007,58(2):171-177.
[35] Granado- Lorencio F,López- López I,Herrero- Barbudo C,et al. Lutein-enriched frankfurter -type products:phys- icochemical characteristics and lutein in vitro bioaccessi- bility[J]. Food Chem,2010,120(3):741-748.
[36] Ye Wenkun, Guo Mingjin, Yi Yuyan, et al. Preparation process research of lutein oil suspension [J]. Journal of Fuzhou University (Natural Science Edition), 2012, 40(3): 418-422.
[37] Li Sen, Guo Xiaoran, Xiang Wenjuan, et al. Preparation and in vitro dissolution study of lutein solid dispersion [J]. Food and Drugs, 2011, 13 (11): 396-399.
[38] Zhang Tianye, Wang Yao, Li Meng, et al. Preparation and in vitro release study of lutein ophthalmic temperature-sensitive in situ gel. Chinese Journal of Pharmacy, 2015, 50 (21): 1880-1884.
[39] Mitri K,Shegokar R,Gohla S,et al. Lipid nanocarriers for dermal delivery of lutein: preparation,characterization, stability and performance[J]. Int JPharm,2011,414(2): 267-275.
[40] Tan Chen. Research on carotenoid liposomes [D]. Wuxi: Jiangnan University, 2015: 1-85.
[41] Wang Chuang, Song Jiangfeng, Li Dajing, et al. Research on lutein microencapsulation [J]. Food Science, 2011, 32 (2): 43-47.
[42] Moraes M,Carvalho JMP,Silva CR,et al. Liposomes encapsulating beta- carotene produced by the proliposomes method:characterisation and shelf life of powders and phospholipid vesicles[J]. Int J Food Sci Technol, 2013,48(2):274-282.
[43] Yang Shuoye, Guo Yun, Chen Xijing. Research progress in the application of new preparation technologies in water-insoluble drugs [J]. Chinese Pharmacy, 2011, 22 (13): 1228-1231.
[44] Zhang P,Omaye ST. Antioxidant and prooxidant roles for β- carotene ,α- tocopherol and ascorbic acid in human lung cells[J]. ToxicolIn Vitro,2001,15(1):13-24.
[45] Xia F,Hu D,Jin H,et al. Preparation of lutein prolipo- somes by supercritical anti-solvent technique[J]. Food Hy- drocoll,2012,26(2):456-463.
[46] Hu Bojie, Hu Yanan, Lin Song, et al. Clinical application of lutein and zeaxanthin in diabetic retinopathy [J]. New Progress in Ophthalmology, 2010, 30(9): 866-868.
[47] Xia Liying, Liu Weijia, Kou Qiuai, et al. Clinical study on lutein for the treatment of age-related macular degeneration [J]. World Traditional Chinese Medicine, 2013, 8(5): 517-519.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese







