How to Extract and Examine Lutein?
1 The current research and industrialization status of lutein
Lutein is a natural plant pigment that not only has a coloring effect, but also has antioxidant and immunity-enhancing effects in humans and animals, and can effectively prevent macular degeneration[1] and cancer[2]. Lutein has been widely used in medicine, health products, food, cosmetics, feed and other fields[3]. Marigold is the most important raw material for extracting lutein [4]. Marigolds are divided into ornamental marigolds and pigment marigolds. The orange-yellow pigment marigolds contain higher levels of lutein [5]. Marigolds are a highly adaptable plant that is easy to grow and has an expectorant effect [6]. At present, the overall traits of domestic marigold varieties are not as good as those of imported varieties [7].
Currently, China accounts for about 90% of the world's marigold cultivation area, and lutein is mainly used in feed at home and abroad. Kemin Food Co. in the United States produces a functional beverage containing lutein [8]. Currently, China's Zhongjin Natural Pigments Group Co., Ltd. and Meikeer (Beijing) Biotechnology Co., Ltd. are developing lutein capsules advancing into the health food sector; Shanxi Hengkang Dairy Company's subsidiary Tiancheng Biotechnology Company[9] uses lutein extracted from marigolds as an additive in chicken feed, and has cultivated marigolds on a large scale locally, forming a local ornamental tourist-oriented agriculture; in 1998, Qingdao University students produced a relatively pure lutein product[10] and obtained a patent for the production of lutein using supercritical CO2 extraction technology.
2 Extraction methods for lutein
Currently, the main extraction methods for lutein are drying, extraction, microwave extraction, maceration, ultrasound, and enzyme-assisted extraction.
2.1 Drying method
A new type of rotary drum dryer is used to pound and dry marigolds to obtain lutein. When the beating ratio is different, the beating efficiency fluctuates between 70% and 90%. The amount of lutein depends on the drying time; when the drying time is the same, the lutein content obtained by drying at 60 °C is higher than that obtained by drying at 70 °C [8].
2.2 Extraction method
The extraction of lutein powder from marigolds has been industrialized. The method of extracting lutein from marigolds using supercritical CO2 is to use marigold flowers as raw material after fermentation, drying, and crushing, and then use supercritical CO2 with ethanol as an entraining agent to extract marigold extract. The marigold flower extract is saponified with potassium hydroxide to obtain a water-soluble natural food coloring lutein resin [8]. Li Gaofeng et al. [11] extracted lutein from marigolds by supercritical CO2 extraction. The highest extraction rate of lutein was obtained when the extraction pressure was 45 MPa, the temperature was 50 °C, the pressure in separation I was 8 MPa, the temperature in separation I was 55 °C, and the temperature in separation II was 20 °C. Xu Pingru et al. [12] used supercritical CO2 extraction to extract lutein from marigolds with a yield of 8.24 mg/g.
2.3 Microwave extraction method
Microwaves are a form of instantaneous penetrating heating. The action of the microwave field breaks down the plant cell walls, thereby accelerating the extraction rate and effectively increasing the product yield [13]. Xu Xia et al. [14] used a microwave method to extract lutein from marigold particles, using petroleum ether as the extraction solvent. When the microwave power was 240W, the material liquid ratio was 1:25, and the extraction time was 45s, the obtained lutein yield was 13.43mg/g. Li Jianying et al. [15] used microwave extraction to extract lutein from citrus peel and tea. 6# solvent oil was selected as the best extraction solvent. For citrus peel, when the microwave power is 800W and the extraction time is 25s, the extraction efficiency of lutein from citrus peel is the highest, at 71.6%; for tea, when the microwave power is 680W, the material liquid ratio is 1:25, and the extraction time is 30s, the highest lutein extraction rate is obtained, at 65.45%.
2.4 Extraction method
The process mainly includes raw material pretreatment, crushing, extraction, filtration, concentration, drying, etc., and the solvent used varies depending on the pigment and raw material [16]. Xia Shulin et al. [17] used tetrahydrofuran as the extraction solvent to obtain crude lutein, which was crystallized to obtain 97.6% pure lutein. Li Xiaomin et al. [16] found that the best solvent for extracting lutein from dried citrus peel was tetrahydrofuran, with a temperature of 60°C and a time of 72 hours, and the lutein content of the extract was more than 50%.
2.5 Ultrasonic method
Ultrasound can generate and transmit powerful energy. When high-energy ultrasound is applied to a liquid, the liquid will be torn into many small cavities when it is in a dilute state. These cavities close in an instant, generating instantaneous high pressure when they close, i.e., the cavitation effect. The cavitation effect of ultrasound generates extremely high pressure, which causes the cell walls of the crushed material and the entire organism to break, and the entire crushing process is completed in an instant. At the same time, the vibration effect of ultrasonic waves enhances the release, diffusion and dissolution of intracellular substances, which is beneficial to the extraction of effective ingredients in the cells [18].
Dai Gang et al. [18] used an ultrasonic method to extract lutein esters from marigolds. The extraction solvent was 80% hexane; the temperature was 30°C, the material-to-liquid ratio was 1:30, and the time was 20 min; the extraction rate was 93.9% when the ultrasonic power was 800 W, which was 24.7% higher than the conventional extraction rate. In order to explore the extraction process of lutein from marigolds, Ye Zhaowei et al. [19] used the water bath heating method and the lutein yield by ultrasonication as the standard to determine the optimal extraction method. The results showed that the extraction of lutein by ultrasonication was significantly better than the other two methods, and the content could reach 21.91 mg/g.
2.6 Enzyme-assisted extraction method
Since enzymes can destroy the structure of cells, the substances inside the cells are more exposed during extraction, and the permeability of the oil is increased [13]. Li Xiuxia et al. extracted lutein from corn protein by enzyme-assisted extraction method. The optimal parameters were enzyme concentration 7682U/g, substrate concentration 8.8%, enzyme digestion time 2.2h, lutein yield 64.65ug/g [20]. Matoushek [21] showed that marigolds were pretreated with cellulase for a period of time, and then extracted and saponified with an organic solvent. The lutein yield was 33% higher than that of the enzyme-free control group.
2.7 Advantages and disadvantages of lutein extraction methods
(1) Extraction method: Compared with the traditional organic solvent method, the use of supercritical CO2 extraction to obtain lutein has the advantages of no solvent residue, preventing the toxicity of the extraction process to the human body and environmental pollution. CO2 gas is cheap and easy to obtain, non-toxic, safe, low cost, easy to separate, and fast to extract. This method has the advantages of simple process, low energy consumption, environmental protection, high product purity, positive hue, good heat and light resistance, and stable color.

(2) Extraction method. Due to the long extraction time, lutein will be destroyed at higher temperatures and undergo oxidative degradation [22].
(3) Ultrasonic method. The extraction of lutein using the ultrasonic method can greatly improve its extraction efficiency. Under the same other experimental conditions, the ultrasonic method and microwave method have the characteristics of short extraction time and high extraction efficiency. The ultrasonic method can fully dissolve the lutein in marigold flowers, increase the dissolution rate, shorten the extraction time, and has high extraction efficiency. It eliminates the impact of high temperatures on the extracted ingredients, saves solvent consumption, and has gradually replaced traditional extraction methods.
(4) Enzyme-assisted extraction method. The enzyme-assisted extraction method uses cellulase to treat the cell wall, destroy the structure of the cell wall, and allow the organic solvent to quickly enter the cell, facilitating extraction [23]. The enzyme-assisted extraction method can improve the extraction rate of lutein because the enzyme medium is active. Compared with the organic solvent extraction method, most of the researchers of this method have conducted research in the laboratory, and large-scale industrial applications are lacking [23]. At present, researchers still need to study whether the cellulase has an impact on the purity of the product when removing the cell wall.
3 Lutein determination methods
3.1 High-pressure liquid chromatography for lutein determination
High performance liquid chromatography (HPLC) is a branch of chromatography technology that has developed from classical liquid chromatography. HPLC is a sensitive, efficient and rapid separation method that can accurately detect the content of various components in a sample. It is currently the most widely used method for quantitative analysis of lutein [24].
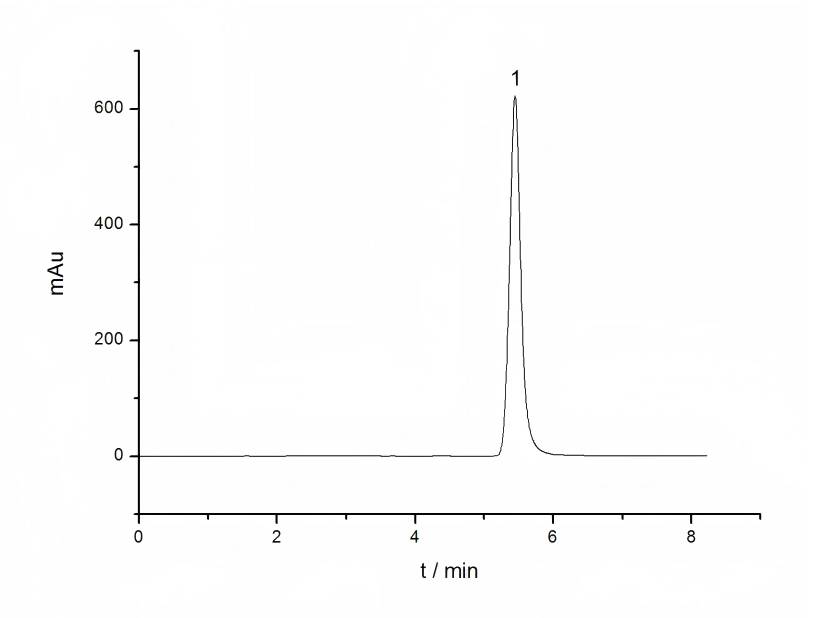
Zhang Guanshun et al. [25] used high-pressure liquid chromatography to determine the lutein content, using acetonitrile: methanol (95:5) as the mobile phase. The lutein solution has the best absorption effect at a wavelength of 446 nm. Chen Jieli et al. [26] used acetonitrile: methanol (98:2) as the mobile phase, with a flow rate of 0.8 mL/min, The column temperature was 25°C, the injection volume was 20μL, lutein was dissolved in 95% ethanol, and the scan showed that the absorption rate was highest at 446nm. This laboratory determined the lutein content in the pigment marigold by high-pressure liquid chromatography. A Shimadzu ODS-3 C18 column (Φ5μm4.6 × 250mm) was used as the chromatographic column, with V (acetonitrile): V (chloroform) = 92:8 as the mobile phase. The detection wavelength was 450nm, flow rate: 1.5 mL/min, detection temperature: 35°C, injection volume: 10 μL, and the lutein content detected was 0.7% to 2.4%.
3.2 Colorimetric method
Colorimetry is based on Lambert–Beer's law (ultraviolet–visible spectrophotometry). Ultraviolet–visible spectrophotometry (UV–vis) is a simple, fast and low-cost method for accurate analysis based on the proportionality between the concentration of the substance being measured and the absorbance [27]. Li Gaofeng [28] improved the lutein determination result by 33% by adjusting the eluent hexane: acetonitrile: methanol ratio to 70:25:5. However, the inability to analyze different isomers of lutein, poor sensitivity, and susceptibility to interference remain prominent disadvantages of ultraviolet-visible spectrophotometry, which severely restricts its application and development.
3.3 Advantages and disadvantages of lutein determination methods
High-pressure liquid chromatography (HPLC) can be used to separate various carotenoid isomers [27]. High-pressure liquid chromatography (HPLC) is a method for determining lutein that is accurate and stable, and can efficiently and quickly detect the content of components in a sample. It is suitable for determining the content of lutein. High-pressure liquid chromatography (HPLC) is highly specific and accurate, but it takes a long time. UV-Visible Spectrophotometry is fast, but has poor sensitivity, is susceptible to interference, has weak specificity and is not accurate enough. The lutein content in marigolds was determined using UV-Visible Spectrophotometry. Compared with other existing methods, it has the advantages of being quick and easy to operate, low detection cost and low solvent consumption. It is suitable for the detection of the total lutein content in marigolds and their crude and refined processed products.
4 Functions of lutein
Vegetables, fruits and carrots are rich in lutein, with marigolds having the highest lutein content [24]. Lutein can delay the weakening of vision and blindness caused by macular degeneration in the elderly. Lutein also has good coloring [31], anti-cancer and anti-oxidation functions. It has been listed as a food coloring and nutrient in Europe and other countries [29].
4.1 Coloring function
Lutein can change the body color of animals, improve the fertilization and hatchability of eggs, and increase the reproductive rate. The coloring of lutein can be used in egg yolks and poultry and chicken feed. Since people tend to prefer yellow to yellow for the nutritional value of chicken, lutein's good coloring effect has been widely used as a feed additive in countries around the world. Lutein is a natural pigment with no side effects. It also meets market demand and health standards because of its safe and nutritious properties [30-31].

4.2 Anti-cancer function
Lutein is one of the main carotenoids in human blood and has special biological functions in inhibiting the growth of tumors [32-33]. Recent studies have shown that lutein can inhibit the growth of various cancers, such as breast cancer, colon cancer, and skin cancer. Research conducted by the New York University School of Medicine has shown that there is a close relationship between breast cancer incidence and lutein intake. The incidence of breast cancer in the experimental group with low lutein intake was 2.08 to 2.21 times higher than that in the group with high lutein intake [34-35].
4.3 Prevention of vision loss
Lutein can effectively prevent age-related macular degeneration (AMD) and cataracts [36]. Regular lutein intake can effectively prevent the harmful effects of computer radiation on the human body [37]. Studies have shown that the macular pigment in the human eye is composed of two types of lutein found in the diet, lutein and zeaxanthin. In patients aged 60 to 81 with age-related macular degeneration, clinical studies have shown that after 15 weeks of lutein supplementation, the macular pigment content has increased significantly, repairing damaged retinal tissue [38]. Therefore, lutein supplementation can significantly improve age-related macular pigmentation and prevent macular degeneration.
4.4 Antioxidant function
Lutein is safe and non-toxic, has antioxidant properties, and is widely used in medicine [39]. Reactive oxygen species in the human body are an important factor that damages human health. Lutein, as an antioxidant, can inhibit the activity of reactive oxygen species and prevent it from damaging normal cells. Lutein can protect the body from harm by quenching singlet oxygen and capturing active oxygen radicals through physical or chemical quenching [40]. Recent research results show [41] that the natural antioxidant lutein can prevent skin damage caused by the sun's harmful rays. Therefore, adding a certain amount of lutein to food can prevent a series of diseases caused by organ aging in the human body. Lutein can also be used in cosmetics to prevent skin damage.
5 Problems and prospects for development
5.1 Problems
Green vegetables, fruits and flowers are all rich in lutein, but marigolds have the highest lutein content [42]. Marigolds are divided into pigment marigolds and ornamental marigolds, and lutein is generally extracted from pigment marigolds. Marigold seeds are now mainly imported from abroad [42]. Some foreign companies are at the forefront of research and development and application. Although China is also constantly carrying out research on lutein, there is still room for improvement compared with foreign countries.
At present, the lutein content of China's pigment marigold varieties is relatively low, and they have poor resistance to black spot disease. Black spot disease can form brown spots on marigold leaves, which continue to spread, eventually causing the leaves to wither and die. Within 7 days, it can quickly cause the entire plant to die, severely restricting marigold production. At present, there is a lack of disease-resistant varieties at home and abroad. The latest research results from the Beijing Agricultural Biotechnology Research Center of the Beijing Academy of Agriculture and Forestry Sciences show that the disease index of the main cultivar of pigment marigold in the field is as high as 76.29. The disease index of some other cultivars is also generally high, with the best being 56.67 and the rest being above 70. This indicates that pigment marigold cultivars resistant to black spot are very scarce in the domestic market. At present, lutein production in China involves processing marigold flowers into dried granules, which are then processed into lutein extract or low-lutein powder for sale. It is used as a raw material for users' further processing or as a feed colorant [43]. The production technology for preparing high-content, high-purity lutein for use in food and medicine is not yet mature, and many research institutes are still developing it. Therefore, efficient and safe lutein extraction and purification technology needs to be improved.

5.2 Development prospects
Lutein is a natural pigment with good coloring power, nutritional and health benefits, and is widely used in food, medical and feed additives. Lutein can also prevent macular degeneration, tumors, cardiovascular disease, and enhance the body's immune system. It will have broad prospects in the food and medical fields. Lutein received GRAS certification from the US Food and Drug Administration in 2002. Lutein and lutein esters are very popular in the US market [44].
Due to its natural coloring ability and its dual nutritional and health effects, lutein has great development and application potential [20]. 1 g of lutein is worth the same as 1 g of gold [6], and it has been given the reputation of “plant gold”. Currently, there is a global market shortfall of about 1 million tons of lutein [45]. Therefore, there is a huge market prospect for the industrial production of marigolds and the extraction of lutein. Every year, enterprises and factories continue to increase the production of marigolds.
Based on the market prospects and commercial value of lutein, the development and production of varieties with independent intellectual property rights is the sustainable development path of the marigold industry, which is of vital importance for the industrialization promotion and brand building in China. In the future, we should increase efforts to cultivate pigment marigold varieties with high lutein content and high resistance to black spot disease. At the same time, we should also develop efficient and safe lutein extraction processes and lutein products, broaden the application fields of lutein, and make it better serve the medical, food, and health care fields.
References
[1] Li Yongxiang, Cao Duanlin. Research progress on the extraction and application of lutein [J]. Shanxi Chemical Industry, 2004, 24(1): 17-18.
[2] Sun Zhen, Yao Huiyuan. The anticancer effect of lutein and the current research status [J]. Biotechnology Newsletter, 2005(1): 84-86.
[3] Wu Zhigang, Wang Ping, Lv Shuangshuang, et al. Research progress on pigmented marigolds [J]. Liaoning Agricultural Sciences, 2007 (4): 33-37.
[4] Meng Zhe, Liu Hongyun. Introduction to lutein [J]. Chemical Education, 2007 (3): 3-4.
[5] Li Dajing, Liu Chunquan, Fang Guizhen. Determination of lutein and lutein ester content in marigold flowers of different strains [J]. Forest Chemical Industry, 2007, 27(2): 106-108.
[6] Huang Yuling. Research on marigold cultivation technology and lutein extraction process [D]. Fuzhou: Fujian Agriculture and Forestry University, 2013.
[7] Li Na, Wang Ping, Wu Zhigang, et al. Research status and development prospects of pigmented marigolds [J]. Northern Horticulture, 2010 (10): 228-231.
[8] Xu Xiulan, Zhao Guohua, Chen Jianquan, et al. Research progress of lutein [J]. Cereals, Oils and Fats, 2004 (10): 3-7.
[9] Huo Bingyin. 40 million mu of marigold flowers are as red as fire [N]. Shanxi Life Morning News, 2002-08-20.
[10] Li Yongxiang, Cao Duanlin. Research progress in the extraction and application of lutein [J]. Shanxi Chemical Industry, 2004, 24 (1): 17-18.
[11] Li Gaofeng, Nie Yongliang, Wang Peiwei. Process optimization of supercritical CO2 extraction of lutein from marigold flowers [J]. Modern Chemical Industry, 2009, 29 (2): 182-186.
[12] Xu Pingru, Yang Zhonglin, Shao Youyuan. Optimization and control of process conditions for supercritical CO2 extraction of lutein [J]. Chemical Technology and Development, 2011, 40 (8): 5-7.
[13] Wang Yanbo, Shi Yan, Tang Huian. Research progress on the extraction, efficacy and application of lutein [J]. China Brewing, 2011 (7): 1-5.
[14] Xu X. Study on the nature of the extraction agent for lutein in marigolds [D]. Wuxi: Jiangnan University, 2005 (9): 18-20.
[15] Li J Y, Deng Y. Study on the microwave extraction method of lutein [J]. Food Additives, 2004, 25 (8): 121-124.
[16] Li Xiaomin. Study on the extraction of lutein from orange peel by extraction method [J]. Anhui Agricultural Science, 2009, 37(11): 4851-4853.
[17] Xia Shulin, Ji Benhua, Ma Bingpeng, et al. Study on the extraction process of lutein from marigold flowers [J]. Anhui Agricultural Science, 2006, 34(19): 5029-5030.
[18] Dai Gang, Su Ji, Chen Yaping, et al. Study on the extraction of lutein from marigold by ultrasonic wave [J]. Yunnan Chemical Industry, 2010, 37(3): 40-41.
[19] Ye Zhaowei, Li Xun, Liu Zhuming, et al. Comparison of three extraction methods for lutein from marigolds. [J]. Hubei Agricultural Sciences, 2012, 53(4): 875-876.
[20] Li Xiuxia, Han Lujia. Optimization of enzymatic extraction process assisted by luteinase for corn protein powder [J]. Chinese Journal of Cereals, Oils and Foodstuffs, 2009, 24(9): 29-31.
[21] Barzana E, Rubio D, Santamria RI, et al. Enzyme-mediated solvent extraction of carotenoids from marigold flowers (Tagetes erecta L.) [J]. J Agric Food Chem, 2002 (50): 4491-4496.
[22] Cao Guangmei, Zhao Guisen, He Yanli. Lutein's protective effect on vision and extraction process [J]. Food and Drugs, 2012, 14(5): 199-202.
[23] Lv Ning. Extraction and prospects of lutein from marigolds [J]. Hunan Feed, 2016(1): 20-25.
[24] Guo Wei. Optimization of marigold lutein extraction process and separation and purification. [D], Harbin: Harbin Engineering University, 2006.
[25] Zhang Guanshun. Determination of lutein content by HPLC [J]. Strait Pharmacy, 2004, 16 (6): 63-64.
[26] Chen Jieli, Li Feng, Lou Yuyang, et al. Determination of lutein content by high performance liquid chromatography [J]. Guangdong Chemical Industry, 2015, 42 (305): 210-211.
[27] Liang Minhui, Cui Yajuan, He Mei, et al. Research progress in the analysis and detection methods of lutein [J]. Food Industry Science and Technology, 2015, 8: 390-394.
[28] Li G. Extraction technology and analysis method of lutein in marigold flowers [D]. Taiyuan: China Research Institute of Daily Chemical Industry, 2009.
[29] Wu Y, Li S. Properties, functions and applications of natural edible lutein in food processing [J]. Food Science and Technology and Economics, 2015, 40(1): 59-63.
[30] Zhang Hui, Li Tao, Xu Gongshi. A natural food colorant with a promising future—lutein [J]. China Food Additives, 2004 (5): 45-48.
[31] Han Xiuping, Zhang Xuyuan. Research progress of lutein coloring [J]. Anhui Agricultural Science. 2009, 37 (6): 2343-2344.
[32] Ding Jiaxing. Extraction of the edible natural pigment lutein [J]. Gansu Science and Technology, 2003, 19 (7): 97-98.
[33] Meng Xianghe, Mao Zhonggui. Health-promoting function of lutein [J]. China Food Additives, 2003 (1): 17-20.
[34] Jean Soon Park, Boon PChew, Ten SWong. Dietary Lutein from Marigold Extract Inhibits Mammary Tumor Development in BALB [J]. The Journal of Nutrition, 1998, 128(10): 1650-1656.
[35] Guo Zhiyou, Gao Cuiling, Song Ru, et al. Function and application of lutein [J]. Hebei Agricultural Science, 2010, 14(2): 52-53.
[36] Li Dajing, Pang Huili, Liu Chunquan. Research progress on the protective effect of lutein and zeaxanthin on the eyes [J]. Jiangsu Agricultural Science, 2013, 41(9): 1-4.
[37] Sowbhagya H B, Krishnamurthy N, Krishnamurthy N. Natural colorant from marigold - chemistry and technology [J]. Food and Reviews International, 2004, 20(1): 33-50.
[38] You Xin. Lutein and its eye protection function [J]. China Food Additives, 2003, 5: 1-3.
[39] Su Qing, Li Qian, Chen Hao, et al. Research on the antioxidant-prooxidant effect of lutein [J]. Food Industry Science and Technology, 2014, 35(9): 68-71.
[40] Li Haoming. Research overview of lutein from marigold and its physiological functions [J]. China Food Additives, 2001 (4): 31-33.
[41] Yang Lifei, Deng Yu. Preliminary study on the extraction process of lutein [J]. Guangzhou Food Industry Science and Technology, 2004, 79 (1): 45-47.
[42] Song Youliang, Wu Dianxing, Qian Guoren, et al. Research progress of lutein [J]. Agricultural Science and Technology Communication, 2013, 11: 138-140.
[43] Wu Xinzhuang, Zhang Hua, Wang Xiaoke, et al. Research progress in the preparation of marigold lutein technology [J]. Food Industry Science and Technology, 2012, 33 (6): 456-459.
[44] O'Donnell, C.D. Function alfutures [J]. Prepared Foods, 2004, 173 (4): 1-4, 6, 8-9.
[45] Lin Dengui, Zeng Li, Wang Peng, et al. Research status and development trend of marigold [J]. Shanghai Agricultural Journal, 2014, 30(6): 145-149.
[46] Yu Xiaoli. Lutein product research [J]. Inner Mongolia Petrochemical Industry, 2005(5): 2-3.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese







