How About the Immunity Benefit of Beta Glucan?
As a dietary fiber with multiple physiological functions, β-glucan cannot be broken down and metabolized by digestive enzymes encoded by human genes. When it enters the body, most of it is fermented and metabolized by intestinal flora, producing a variety of metabolites that promote human health, such as short-chain fatty acids (SCFAs) (acetic acid, propionic acid, butyric acid, etc.). As host signal molecules and energy substrates, they can regulate immune cells and promote the intestinal barrier through various ways to regulate immune cells and promote the intestinal barrier [1‒2]. At the same time, β-glucan, as a prebiotic, can maintain and restore the balance of intestinal probiotics, reduce the body's energy intake, and prevent and treat obesity, cardiovascular disease, cancer, diabetes, etc. [3‒5]. Numerous studies have shown that β-glucan from different sources, types and degrees of polymerization can regulate many metabolic processes and the development of diseases in the human body through indirect or direct pathways, exerting a variety of beneficial effects [6].
The intestine is one of the body's tissues and organs that come into closest contact with the external environment, and also an important barrier that defends against external pathogens entering the body [7]. The intestinal immune system mainly includes the innate immune system, adaptive immune system and mucosal immune system. The intestinal mucosa is the main site of contact between the body and antigens and pathogens. Intestinal epithelial lymphocytes are the mainstay of intestinal immunity [8]. The intestinal immune system is composed of immune cells, immune molecules and intestinal flora, and the three interact to regulate intestinal immunity [9]. For example, changes in the intestinal flora can cause changes in the proliferation and differentiation of immune cells and changes in cytokine secretion [10].
Dendritic cells (DCs) are responsible for antigen and cytokine presentation, maintaining a balance between effector T cells and regulatory T cells (Tregs). Tregs can inhibit the body's excessive cellular immune response to the intestinal flora, assist in the colonization of probiotics, and promote the differentiation of helper T cells (Th) and B cells [1 1], which is a key way for the intestinal mucosal system to maintain its own stability. Intestinal homeostasis is an important factor in maintaining a good immune system and good health.
However, with the accelerated pace of life, the emergence of various processed foods and irregular eating habits, the intestinal homeostasis of the body is more likely to be disrupted, and it is more likely to cause various related diseases [7]. A high-dietary-fiber diet can increase the release of SCFAs in the intestine, reshape the intestinal flora, and improve the integrity of the intestinal barrier, thereby regulating intestinal immunity [12]. This paper discusses the role and mechanism of β-glucan in enhancing intestinal immunity from multiple perspectives, including the intestinal barrier, intestinal flora and intestinal cells. It further reviews its biological significance, with the hope of providing theoretical guidance for further research on the functional network of β-glucan and the body's intestinal interaction, and providing a theoretical basis and ideas for in-depth research on the role and mechanism of β-glucan in promoting body health.
1. Source and characteristics of β-glucan
Beta-glucan is a non-starch polysaccharide composed of D-glucose monomers linked by β-glycosidic bonds. It can be divided into two categories based on solubility: water-soluble and insoluble. Its solubility mainly depends on its molecular weight, and generally, beta-glucan with a molecular weight greater than 100 Da is essentially insoluble [13]. Beta-glucan is widely available, including grains, fungi, bacteria, seaweed . Cereals, yeast and mushrooms are the three main sources of β-glucan [14]. Cereal β-glucan is a soluble dietary fiber that is usually a linear homopolymer composed of β-D-glucose linked by continuous β-1,4 and occasional β-1,3 glycosidic bonds. It is abundant in the cell walls of the endosperm and aleurone layers of oat and barley grains [15]. Yeast β-glucan is composed of β-1,3 glycosidic bonds as the main chain and β-1,6 glycosidic bonds as the side chains [16]; the composition of mushroom β-glucan is the opposite of yeast β-glucan, with β-1,6 glycosidic bonds as the main chain and β-1,3 glycosidic bonds as the side chains [17 ].
The three main sources of β-glucan have different structures and physicochemical properties, and their biological activities also differ. In addition, as research on β-glucan progresses, so too does the development of extraction methods. Traditional methods of β-glucan extraction include pressurized hot water extraction, acid extraction, alkali extraction, enzyme extraction and mixed extraction; new extraction methods include ultrasound-assisted extraction and microwave-assisted extraction [18]. Hot water extraction is mainly used for soluble β-glucan, and the temperature is controlled at 47-50°C for best results. However, this method is inefficient and time-consuming. Alkaline or acidic extraction can lead to degradation of β-glucan, which can easily damage its structure and affect its biological activity. Although enzymatic extraction can overcome these problems, it is costly and has strict reaction conditions. In contrast, ultrasonic-assisted extraction and microwave-assisted extraction have many advantages such as being simple and efficient [19‒20].
Different sources and extraction methods of β-glucan will have different effects on the structure of β-glucan, which in turn affects its biological activity. The structure-activity relationship of β-glucan has also received increasing attention in research. Structure determines properties.
The biological activity of β-glucan is affected by its solubility, molecular weight, molecular conformation, degree of branching, etc. Each factor affects its biological activity to a different extent, with the main chain structure (type of glycosidic bond, mode of monosaccharide attachment) being the main factor, the degree of branch
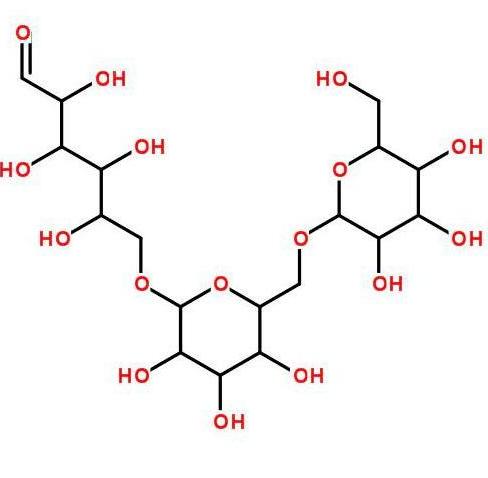
ing (presence or absence of branching) being second, and the monosaccharide composition (type of monosaccharide in homo- or hetero-glucan) having the least effect [21‒22]. Studies have found that compared to the low molecular weight β-glucan from Agrobacterium ZX000, the high molecular weight β-glucan has a higher anti-inflammatory activity, and it is speculated that this difference in efficacy may be related to Dectin-1 [23]. The water-soluble β-glucan derived from the enzymatic hydrolysis of yeast glucan induces dynamic morphological changes and enhanced phagocytic activity in RAW264.7 macrophages. It has been experimentally proven that β-1,3-glucan without any side chains cannot activate phagocytes [24]. HUANG et al. [25] elaborated on the structure-activity relationship of natural glucans in detail.
2. The immunomodulatory pathways and mechanisms of 1-dextan-based intestinal
On the one hand, the intestine is the main site of absorption and metabolism of β-glucan, and it is also the largest site of contact with pathogens. On the other hand, β-glucan can improve the intestinal environment and enhance intestinal immune function [7]. Nearly half of the body's immune cells are distributed in the intestine, and they form an intestinal immune system with the intestinal flora [26]. Loss of intestinal barrier function can lead to systemic immune imbalance and trigger immune diseases [27]. When the intestinal barrier is damaged, intestinal permeability increases and a large number of pathogens enter the body. Immune cells in the intestine, such as neutrophils, macrophages, and lymphocytes, are recruited or activated, which increases the ratio of two different Th functions in the systemic circulation, namely Th2/Th1, and increases the secretion of pro-inflammatory cytokines, further destroying the integrity of the intestinal barrier and imbalancing the intestinal flora [8]. Beta-glucan acts on the intestinal flora and immune cells by directly or metabolically producing SCFAs through the intestinal flora, restores and maintains intestinal flora balance, improves intestinal barrier integrity, thereby maintaining intestinal homeostasis, enhancing intestinal immunity and the nutritional health of the body.
2.1 Beta-glucan improves intestinal barrier function
The intestinal barrier includes biological, chemical, mechanical and immune barriers [28]. Damage to the intestinal barrier is a key driver of the entry of a large number of external pathogens into the body, and beta-glucan can improve intestinal barrier integrity. First, tight junction proteins and mucins are the main components of the intestinal barrier. Beta-glucan can protect the intestinal mucosal barrier by upregulating the expression of tight junction proteins (occludin, ZO, etc.) in intestinal epithelial cells [29]. One of the main intestinal metabolites of beta-glucan, butyrate, can promote the extracellular assembly of tight junction proteins through the mitogen-activated protein kinase (AMPK) pathway promote the extracellular assembly of tight junction proteins without affecting the expression of tight junction proteins, and can intervene to relieve intestinal inflammation and enhance intestinal barrier function by the TLR4-NF-κB pathway, thereby reducing intestinal barrier permeability [30].
Secondly, it can also inhibit bacterial growth by forming antimicrobial peptide Reg IIIγ, thereby protecting the intestinal biological barrier. It can also increase the expression of mucin in intestinal mucosal epithelial cells and the activity of secreted enzymes in intestinal mucosal epithelial cells, thereby strengthening the chemical barrier effect of the intestinal mucosa [31]. In one study, rainbow trout fed a diet supplemented with β-glucan and a commercially available functional diet were found to have an increased number of goblet cells in the distal intestine. Cup cells are specialized epithelial cells that secrete important antimicrobial peptides, mucins and cytokines, and are important for maintaining the integrity of the intestinal barrier [32]. Finally, SCFAs can regulate the immune barrier of the intestinal mucosa by activating G-protein-coupled receptors (G-protein-coupled receptors, GPCR) and inhibiting histone deacetylase (HDAC) [33]. An in vitro study demonstrated that β-glucan can regulate the expression of the circadian rhythm regulator 2 protein through its final intestinal metabolite ethanol, thereby affecting the permeability of the intestinal barrier [34].
Lymphocytes are the main immune cells in the intestine, such as DC, lymphoid B cells, and lymphoid T cells [35]. Beta-glucan can improve intestinal barrier function by inducing DC autophagy, promoting the activation and differentiation of Th1 and cytotoxic T lymphocytes, inhibiting the secretion of inflammatory cytokines, and stimulating intestinal epithelial regeneration [36]. In addition, butyrate can stimulate B cell differentiation to produce IgA and IgG, enhancing the body's immune system [37]. In addition, DCs and macrophages have specific phagocytic receptors on their surface that bind to β-glucan (common specific phagocytic receptors can be divided into direct and indirect β-glucan recognition receptors, the main ones being Dectin-1, SR, Langerin and CR3). When the two bind together, they can activate intracellular signal transduction pathways, initiate phagocytosis as soon as they are attacked, promote the release of cytokines, and regulate the body's intestinal immunity[ 38].
2.2 Bifidobacterium-glucan balances the intestinal flora
The intestinal flora, as part of the intestinal barrier and intestinal immune system, is an important factor in maintaining intestinal and body health [10]. Current research suggests that a high-fiber diet is a means of shaping the intestinal flora [39]. In addition to Bifidobacterium and Lactobacillus, which are the main probiotics in the human intestine, the genus Bacteroides is also an important keystone genus in the intestine [40]. Studies have found that the genus Bacteroides has outer membrane proteins that bind to β-glucan on its surface, and that Bacteroides in the intestine can encode many β-glucan lyases and glycoside hydrolases to metabolize and degrade β-glucan [41].
β-glucan can selectively stimulate the growth of intestinal flora, changing the composition, abundance and diversity of the intestinal flora [42]. For example, oat β-glucan can stimulate the proliferation of bifidobacteria and lactobacilli in the mouse intestine, while inhibiting the growth of Escherichia coli, effectively improving the intestinal environment of mice[43]. It can also significantly increase the content of Bacteroides in the intestines of obese mice, and reduce the content of Adlercreutzia equolifaciens, Bacteroides intestinalis and Pept ostreptococcaceae noname, etc., and increase the number of beneficial bacteria such as Bacteroides dorei, Bacteroides xylanisolvens and Parabacteroides distasonis, etc., to regulate the intestinal flora and improve intestinal health [44]; supplementing the pig diet with yeast β-glucan can change the α diversity and β diversity of its fecal flora [45].
In addition, there are hundreds of millions of microorganisms living in the intestine that can participate in the protection of intestinal health. Significant changes in the intestinal flora can regulate the development of immune cells, thereby regulating intestinal homeostasis and immunity [12]. When the body is in a healthy condition, Th1 and Th2 are in balance. However, when the body develops some immune diseases such as food allergy (FA), the balance between the intestinal flora and various immune cells such as DC, Th1 and Th2 is imbalanced [39]. For example, a study transplanted the intestinal microbiota of human milk-allergic patients into germ-free mice, which failed to inhibit the induction of allergic reactions, but the microbiota of healthy people and FA-resistant wild mice had a protective effect [46], indicating that the intestinal flora of healthy people and patients is significantly different and can significantly affect the intestinal and body immune functions.
An important factor in the ecological regulation of the immune response by the intestinal flora is Treg cells [47]. Clostridium and Bacteroides can promote Treg cells and their function and induce the production of key anti-inflammatory factors [48]. This is manifested by upregulating the expression of recombinant human transforming growth factor (TGF), inducing the proliferation and differentiation of intestinal Treg and Th17, as well as the secretion of cytokines such as interleukin (IL)-17 and IL-6, inhibiting immunoglobulin IgE, upregulating IgA, and enhancing immune function [48–50]. It also stimulates NK cells to secrete IL-22, which enhances the body's mucosal immune response [51]. This is mainly dependent on the activation of the MyD88-dependent microbial sensing pathway in the commensal bacteria in the original Treg cells, thereby producing inhibitory ROR-γt Treg cells [52]. Of course, the effect of the intestinal flora on the host is not limited to direct action. It can also regulate stem cells through immune signals, thereby leading to permanent changes in the intestinal environment [53].
Finally, there is a two-way interaction between the intestinal flora and intestinal mucus. The intestinal mucosa provides a long-term suitable living environment for microorganisms, and in turn, microorganisms maintain the integrity of the intestinal barrier through intercellular connections and promote the repair ability of the intestinal epithelium [54–55]. The mucus layer is an important barrier in the intestine. Mucus-loving bacteria and adherent lactobacilli have been shown to help increase the expression of mucin genes and are key intestinal flora that modify the mucus layer [56].
When β-glucan alters the composition and ratio of intestinal flora, the intestinal mucus barrier also changes. Intestinal flora can also enhance barrier function by activating type III innate lymphoid cells to change epithelial gene expression, with Treg playing an important role in maintaining intestinal barrier integrity and flora homeostasis [57]. In summary, β-glucan intervention can promote intestinal barrier function, improve intestinal microbial dysbiosis, and enhance intestinal immune function.
2.3 The immunomodulatory mechanism of β-glucan's main intestinal metabolite SCFAs
The prerequisite for exerting a series of biological activities is digestion and absorption. SCFAs are transported in the intestine by diffusion in the form of fat-soluble substances [58]. However, research on the mechanism of β-glucan absorption in the intestine is still incomplete. Current research suggests that there may be two ways in which β-glucan is absorbed: one is intestinal absorption, in which the absorption of β-glucan is mediated by Dectin-1 receptors on the surface of macrophages and dendritic cells; the other is cellular absorption, which includes the absorption of β-glucan mediated by microfold cells and extracellular matrix cells [59]. Digestion and absorption are prerequisites for the application of β-glucan. Direct digestion and absorption, phagocytosis, and intestinal flora degradation are the three possible mechanisms involved, with intestinal flora degradation being the most important [29].
In vitro gastrointestinal digestion experiments have found that β-glucan is only partially degraded by intestinal hydrolases and gastric acid. Further simulations of the fermentation degradation of β-glucan by intestinal flora have found that β-glucan does undergo most of its fermentation degradation under the action of intestinal flora [60]. It can be said that intestinal flora is an important bridge for the interaction between β-glucan and the human body. Undigested β-glucan enters the intestine and is fermented and degraded by intestinal flora (especially anaerobic bacteria), producing SCFAs, indoles and other products. Current research on SCFAs mainly focuses on acetate, propionate and butyrate, and the main species that metabolize these three SCFAs are different. Among them, acetate is mainly produced by Bifidobacterium through the Wood-Ljungdahl pathway and acetyl coenzyme A [61]; propionate is mainly produced by Bacteroides and Firmicutes through the succinate pathway, and butyrate is mainly produced by Clostridium clusters IV and XIVa through butyrate kinase or the action of butyrate coenzyme A [62]. In subsequent reactions, some propionic acid and butyric acid react further to produce ethanol. Since not all intestinal flora can produce all SCFAs, the ratio and distribution of different types of SCFAs in the intestine can to some extent reflect the intestinal flora.
The main intestinal metabolites of dietary fibres, including β-glucan, are SCFAs. As effective regulators of the mucosal immune system, SCFAs are associated with the induction of immune tolerance [63]. Current research suggests that SCFAs are involved in immune regulation in the gut through three main pathways. The first pathway is through the signaling of metabolites by GPCR. SCFAs bind to the metabolites GPR43, GPR109A and GPR41 expressed on epithelial cells and immune cells with different affinities, which increases the activity of tolerogenic CD103+ DCs, increases the number of Treg cells, and enhances tolerance to commensal bacteria and intestinal barrier function [8].
The second mechanism is that SCFAs (especially butyrate) act as HDAC inhibitors and thus as epigenetic regulators of Tregs [64‒65]. In other words, SCFAs can increase histone acetylation to upregulate the expression of the forkhead box protein P3 (FOXP3) transcription factor in the body, and FOXP3 can induce and maintain the immunosuppressive Treg phenotype. In other words, SCFAs can indirectly regulate Treg differentiation [66]. The third pathway is that SCFAs metabolically consume intestinal oxygen, creating an hypoxic environment that promotes HIF gene expression and enhances intestinal tissue barrier function [67]. It has been reported that excessive reactive oxygen species increase the susceptibility to allergic diseases, and oxidative stress is a key activator of intestinal damage and inflammatory diseases [68]. However, most of the current research on the immunomodulatory function of SCFAs focuses on a single SCFA, and there is a lack of clinical research data. In the future, the combined effects of multiple SCFAs should be considered, and other possible immunomodulatory pathways and mechanisms should be explored in depth.
3. The biological significance of the participation of β-glucan in intestinal immunomodulation
Food allergy (FA) is an abnormal immune response caused by a food allergen and is a type I hypersensitivity reaction. Current animal studies have shown that β-glucan intervention can alleviate food allergy symptoms. For example, adding dietary β-glucan to the daily diet of pigs and mice can increase the number of Clostridium species in the intestine, promote the production of intestinal Treg cells, thereby inhibiting IgE, upregulating IgA expression, and alleviating FA reactions [69–70]. In addition, the intestinal metabolites of β-glucan, SCFAs, can inhibit mast cell activation and reduce the release of inflammatory mediators such as histamine and IL-6 on the one hand, and stimulate B cell differentiation to produce IgA to enhance the body's immunity on the other hand, thereby alleviating the symptoms of FA [71‒72].
In addition, numerous studies have demonstrated the important role of β-glucan in altering glycolipid metabolism, preventing obesity, and fighting cancer [73]. β-Glucan can effectively lower the body's cholesterol level by regulating physiological cholesterol levels and rebalancing, rather than just blocking the action of liver enzymes responsible for producing cholesterol, as statins do [74]. In a rat model, it was found that yeast β-glucan supplementation reversed obesity and intestinal flora changes caused by a high-fat diet, a process that was associated with the participation of β-glucan metabolites. In another study, oat β-glucan was found to inhibit lipogenesis and fat degeneration in mice with hyperlipidemia by downregulating fatty acid synthase and sterol regulatory element binding protein-1, upregulating peroxisome proliferator-activated receptor α and activating the AMPK pathway in the liver and adipose tissue [75].
Insoluble yeast β-glucan can improve the changes in the composition of the intestinal flora and the damage to the intestinal barrier caused by a high-fat diet, increase SCFAs-producing bacteria and reduce obesity-related bacteria, indicating that the intestine plays an important protective role in β-glucan against high-fat-induced obesity [76]. For example, Lv Zhenyue et al. [44] found that oat β-glucan intervention can accelerate fat metabolism, reduce fat accumulation in mice, and control the rate of weight gain in mice. A randomized double-blind study in Japan found that high β-glucan barley can significantly reduce the visceral fat area, body weight, and body fat percentage of patients with metabolic syndrome, effectively preventing visceral fat obesity [77].
GPCR43 plays a key role in regulating glycolipid metabolism and insulin sensitivity. The health benefits of beta-glucan on diabetes have also been widely studied. The pathways involved mainly induce the synthesis of intestinal hormones, hinder the absorption of glucose and lipids, slow gastric emptying and prolong the time of dietary glucose absorption. In this process, β-glucan mainly downregulates glycogen synthase kinase-3 transcription and activates the PI3K, Akt, GSK-3 and GLUT-4 signaling pathways [78]. GUO et al. [79] used an animal model to investigate the effect of oat β-glucan intervention on diabetes in mice. Histological and metabolomics analysis found that oat β-glucan can regulate the levels of total cholesterol cholesterol, low-density lipoprotein cholesterol, and serum amino acids, bile acids and other metabolites, thereby improving diabetes symptoms and alleviating visceral lesions.
The inhibitory effect of β-glucan on cancer and tumors can be attributed to three aspects: first, prevention; second, enhancement of the body's immunity; and third, direct inhibition. β-Glucan plays an important role in anti-tumor by acting on a series of receptors such as Dectin-1 and CD3, and then triggering the release of cytokines such as tumor necrosis factor by some immune cells such as T cells, macrophages, and natural killer cells[80 ]. CHOROMANSKA et al. [81] found that oat low-molecular-weight β-glucan has a strong anti-cancer effect on human skin cancer due to its low molecular weight, high water solubility, and low viscosity.
In addition, its biocompatibility and safety with normal cells make it a promising adjuvant for the treatment of skin cancer. Cancer-related inflammation is generally considered to be a cancer marker. A study found that yeast β-glucan can inhibit intestinal inflammation and reshape the intestinal inflammatory microenvironment to relieve the occurrence and development of colorectal tumors. In the study, azomethine and sodium dextran sulfate were used to induce intestinal inflammation in mice, and it was found that β-glucan intervention can effectively improve intestinal inflammation and delay the occurrence of cancer [82]. Shiitake β-glucan (β-glucan from Lentinus edodes, LNT) has a significant antitumor effect due to its unique triple-helix structure. ZHANG et al. [83] studied the mechanism of LNT's antitumor effect and found that LNT inhibited the accumulation of hypoxia-mediated HIF-77α in a concentration-dependent and Nur77-dependent manner, hindering the growth of breast tumor cells and invasion of lung tissue, and exhibits significant inhibitory effects on breast cancer.
4 Summary and outlook
The intestine is not only the main site for the digestion and absorption of nutrients, but also an important barrier against external pathogens. Maintaining intestinal homeostasis and strengthening intestinal immunity is crucial for maintaining nutritional health. As a dietary fibre, β-glucan has prebiotic properties and is an effective intestinal immune enhancer. It plays an important role in preventing and alleviating diseases such as tumour immunity, diabetes, obesity and fatty acid. Current research on the immunomodulatory effects of β-glucan focuses on its effects on the intestinal flora, intestinal barrier, immune cells and related cytokines. The interaction between the intestinal flora and the body has always been a research hotspot in the field of immunity. The protection of the intestinal barrier integrity by β-glucan is mainly achieved by increasing the expression of tight junction proteins, enhancing the physical and chemical barriers, and reducing intestinal permeability. In particular, for the biological barrier, it regulates the composition of various bacteria composition, increase the ratio of beneficial to harmful bacteria, and maintain a balanced state. The complex interaction between β-glucan and the intestines and the benefits it provides to the body suggest that consuming a diet high in dietary fibre is an effective way to enhance intestinal immune function and improve overall health.
Many studies have reported the ways and mechanisms by which β-glucan promotes intestinal barrier repair and regulates the body's immune system in different research subjects (BALB/c mice, piglets, fish, etc.) and different disease models (lipopolysaccharide-induced colitis, food allergens such as peanut-induced FA, etc.). However, there are few reports in animal experiments on the specific targets or genera of β-glucan immunomodulation, and research on the intestinal flora mainly focuses on intestinal bacteria, with little coverage of other flora. Since SCFAs are not only distributed in the intestine, but may also play a role through other pathways, further research is needed on this aspect.
In addition, β-glucans powder from different sources also differ in their biological activity and mode of action due to structural differences. Future research should combine multi-omics analysis methods, animal experiments and molecular mechanisms to further elucidate the molecular mechanism of the degradation and metabolism of β-glucan by various bacteria in the intestine and the specific targets of its products in regulating the body's immune system. Further cohort studies should be conducted to elucidate the mechanism by which β-glucan enhances the body's immune system by regulating intestinal function and its functional network of interactions with the body, in order to provide a more more targeted and personalized intestinal health action model for clinical β-glucan nutritional intervention to treat related immune diseases, and also provide strategies for the development of β-glucan nutritional health products.
Reference:
[1] SHAH BR, LI BIN, SABBAH HA, et al. Effects of prebiotic dietary fibers and probiotics on human health: With special focus on recent advancement in their encapsulated formulations [J]. Trends Food Sci Technol, 2020, 102: 178‒192.
[2] TAN JK, MACIA L, MACKAY CR. Dietary fiber and SCFAs in the regulation of mucosal immunity [J]. J Allergy Clin Immun, 2023, 151(2): 361‒370.
[3] HAN XY, YANG D, ZHANG S, et al. Characterization of insoluble dietary fiber from Pleurotus eryngii and evaluation of its effects on obesity-preventing or relieving effects via modulation of gut microbiota [J]. Future Foods, 2023, 3(1): 55‒66.
[4] QIN P, HUANG CH, JIANG B, et al. Dietary carbohydrate quantity and quality and risk of cardiovascular disease, all-cause, cardiovascular and cancer mortality: A systematic review and meta-analysis [J]. Clin Nurt, 2023, 42(2): 148‒165.
[5] PENG F, REN X, DU B, et al. Insoluble dietary fiber of pear fruit pomace (Pyrus ussuriensis Maxim) consumption ameliorates alterations of the obesity-related features and gut microbiota caused by high-fat diet [J]. Funct Foods, 2022, 99: 1756‒4646.
[6] HE Y, WANG BX, WEN LK, et al. Effects of dietary fiber on human health [J]. Food Sci Hum Well, 2022, 11(1): 2213‒4530.
[7] HAO XJ, LV HM, ZHANG Q, et al. Research progress of intestinal microbial metabolites mediated intestinal immunity [J/OL]. J Feed Res: 1-16. [2023-09-01]. http://kns.cnki.net/kcms/detail/11.2114.S.20230626. 1512.002.html
[8] LI MM, ZHOU Y, LI YQ, et al. Research progress of the effects of intestinal flora on intestinal mucosal immune system [J]. Chem Vit Sin, 2017, 37(6): 895‒900.
[9] SHA SS, DONG SR, YANG YJ. Research progress of intestinal flora and metabolites regulating host intestinal immunity [J/OL]. Biotechnol Bull:1‒11. [2023-09-01]. DOI: 10.13560/j.cnki.biotech.bull.1985.2022‒1530
[10] LUAN YQ, YANG JX, TAO EL, et al. Research progress on the effects of intestinal flora on intestinal cellular immunity [J]. Chin J Immunol, 2018, 34(11): 1734‒1737, 1742.
[11] FEN ZS, LIU ZJ, WU W. Research progress of intestinal immune regulatory cells in maintaining intestinal mucosal homeostasis [J]. Gastroenterology, 2020, 25(7): 436‒440.
[12] TAN J, MCKENZIE C, VUILLERMIN PJ, et al. Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways [J]. Cell Rep, 2016, 15(12): 2809‒2824.
[13] DU B, BIAN ZX, XU BJ. Effects of natural β-glucan derived from grains and microorganisms on skin health promotion: A review [J]. Phytother Res, 2014, 28: 159‒166.
[14] BAI JY, REN YK, LI Y, et al. Physiological functionalities and mechanisms ofβ-glucans [J]. Trends Food Sci Technol, 2019, 88: 57‒66.
[15] SHOUKAT M, SORRENTINO A. Cereal beta-glucan: A promising prebiotic polysaccharide and its impact on gut health [J]. Int J Food Sci Technol, 2021, (56): 2088‒2097.
[16] BOUTROS JA, MAFEE AS, DONALD C. Comparison of structural differences between yeast β-glucan sourced from different strains of Saccharomyces cerevisiae and processed using proprietary manufacturing processes [J]. Food Chem, 2022, 367: 130708.
[17] KOZARSKI M, KLAUS A, GRIENSVEN LV, et al. Mushroom beta-glucan and polyphenol formulations as natural immune enhancers and balancers: Applied properties [J]. Food Sci Hum Wel, 2022, 12(2): 396.
[18] MAHESHWARI G, SOWRIRAJAN S, JOSEPH B. Extraction and isolation of beta-glucan from cereal sources-A review [J]. Food Sci, 2017, 82: 1535‒1545.
[19] LIU HB, LI Y, YOU ML, et al. Comparison of physicochemical properties of β-glucans extracted from hull-less barley bran by different methods [J].
[20] HE JL, GUO H, WEI SY, et al. Effects of different extraction methods on the structural properties and bioactivities of polysaccharides extracted from Qingke (Tibetan hulless barley) [J]. J Cere Sci, 2020, 92: 102906.
[21] GOUDAR G, SHARMA P, LONGVAH SJ, et al. Effect of processing on barley β-glucan content, its molecular weight and extractability [J]. Int J Biol Macromol, 2020, 162: 1204‒1216.
[22] HAN B, KARTIK B, ERIC C, et al. Structure-functional activity relationship of β-glucans from the perspective of immunomodulation: A mini-review [J]. Front Immunol, 2020, 11: 1664‒3224.
[23] JUN QL, CHEN C, DU YJ, et al. Anti-inflammatory effects of low and high molecular weight β-glucan of Agrobacterium ZX09 in LPs-induced weaned piglets [J]. Food Funct, 2020, 11: 585‒595.
[24] XIN YJ, HYANGGI J, EUNAE C, et al. Immune-enhancing effect of water-soluble beta-glucan derived from enzymatic hydrolysis of yeast glucan [J]. Biochem Biophys Rep, 2022, 30: 101256.
[25] HUANG G, HUANG SX. Structure-activity relationship of natural glucan [J]. Plant Therapy Res, 2021, 35: 2890‒2901.
[26] WANG K, HUANG XT. Research progress of intestinal flora regulation of immune function [J]. J Cell Mol Immunol, 2018, 34(2): 186‒190.
[27] PETERSON LW, ARTIS D. Ntestinal epithelial cels: Regulators of barrier function and immune homeostasis [J]. Nat Rev lmmunol, 2014, 14: 141‒153
[28] WU GH. Intestinal barrier function [J]. Jpen-Parenter Enter, 2004, 11(1): 44‒47.
[29] CHEN CH, HUANG XJ, WANG H, et al. Effect of β-glucan on metabolic diseases: A review from the gut microbiota perspective [J]. Curr Opin Food Sci, 2022, 47: 2214‒7993.
[30] PENG LY, LI ZR, GREEN RS, et al. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers1, 2 [J]. J Nutr, 2009, 139(9): 1619‒1625.
[31] ZHOU WG, WU Y, FENG RT, et al. Highland barley β-glucan boosted intestinal epithelial regeneration via cell cycle and autophagy [J]. Bioact Carbohyd Diet Fibre, 2023, 30: 2212‒6198.
[32] PORTER D, PEGGS D, MCGURK C, et al. In-vivo analysis of Protec™ and β-glucan supplementation on innate immune performance and intestinal health of rainbow trout [J]. Fish Shellf Immunol, 2023, 134:
1050‒4648.
[33] MAJY, PIAO XS, MAHFUZ S, et al. The interaction among gut microbes, the intestinal barrier and short chain fatty acids [J]. Anim Nutr, 2022, 9: 159‒174.
[34] SWANSON G, FORSYTH CB, TANG, YM, et al. The role of intestinal circadian rhythm genes in alcohol-induced intestinal hemophagia [J]. Alcohol Clin Exp Res, 2011, 35: 1305‒1314.
[35] HUANG JM, YANG DX, LI XX, et al. Research progress of intestinal mucosal immunity and inflammatory bodies [J]. Microb Infect, 2019, 14(2): 113‒123.
[36] DING J, NING Y, BAI Y, et al. β-Glucan induces autophagy in dendritic cells and influences T-cell differentiation [J]. Med Microbiol Immunol, 2019, 208: 39‒48.
[37] DI COSTANZO M, DE PAULIS N, BIASUCCI G. Butyrate: A link between early life nutrition and gut microbiome in the development of food allergy [J]. Life, 2021, 11(5): 384.
[38] ZHANG YZ, LIU XN, ZHAO J, et al. The phagocytic receptors of β-glucan [J]. Int J Biol Macromol, 2022, 205: 430‒441.
[39] ZHONG QH, WANG ZL, WU Y, et al. Research progress on the interaction between dietary fiber and intestinal microorganisms in regulating food allergy [J]. Food Sci, 2022, 43(3): 239‒248.
[40] LI C, XING QB, SUN GJ, et al. Study evidence of prebiotic effect of oat/barley β-glucan [J]. Nutrition, 2022, 44(4): 404‒409.
[41] XU MD, MO XX, HUANG H, et al. Yeast β-glucan alleviates cognitive deficit by regulating gut microbiota and metabolites in Aβ1-42-induced AD-like mice [J]. Int J Biol Macromol, 2020, 161: 258‒270.
[42] ZHANG F, FAN DJ, HUANG JL, et al. The gut microbiome: Linking dietary fiber to inflammatory diseases [J]. Med Microecol, 2022, 14: 2590‒0978.
[43] SHEN RL, WANG ZC, YAO HY. Effects of oat β-glucan on intestinal flora in mice [J]. Food Sci, 2005, (2): 208‒212.
[44] LV ZY, MA D, XU HG, et al. Effects of oat β-glucan on obesity and intestinal flora in high-fat diet mice [J]. J Food Saf Qual, 2021, 12(12): 5024‒5030.
[45] LOVING CL, SHAWN MDB, BRADLEY LB, et al. Effect of dietary β-glucan on intestinal microbial diversity and Salmonella vaccine immunogenicity and efficacy in pigs [J]. Vet Microbiol, 2023, 278: 1378‒1135.
[46] FEEHLEY T, PLUNKETT CH, BAO RY, et al. Healthy infants harbor intestinal bacteria that protect against food allergy [J]. Nat Med, 2019, 25: 448‒4543.
[47] RIMA R, STEPHEN-VICTOR E, CHATILA TA. The microbial origins of food allergy [J]. Allergy Clin Immun, 2021, 147(3): 808‒813.
[48] HO H, BUNYAVANICH S. The role of the microbiome in food allergy [J]. Curr Allergy Asthma Rep, 2018, 18(4): 27.
[49] TURNER JA, STEPHEN-VICTOR E, WANG S, et al. Regulatory T cell-derived TGF-β1 controls multiple checkpoints governing allergy and autoimmunity [J]. Immunity, 2020, 52(6): 1202‒1214.
[50] PERONI DG, NUZZI G, TRAMBUSTI I et al. Microbiome composition and its influence on the development of allergic diseases [J]. Front Immunol, 2020. DOI: 10.3389/fimmu.2020.00700
[51] SATOH-TAKAYAMA N, VOSSHENRICH CAJ, LESIEAN-POTTIER S, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+cells that provide innate mucosal immune defense [J]. Immunity, 2008, 29(6) :958‒970.
[52] ABRIL AG, CARRERA M, SANCHEZ-PEREZ A, et al. Gut microbiome proteomics in food allergies [J]. Int J Mol Sci. 2023, 24(3): 2234.
[53] LIU X, NAGY P, BONFINI A, et al. Microbes affect gut epithelial cell composition through immune-dependent regulation of intestinal stem cell differentiation [J]. Cell Rep, 2022, 38(13): 2211‒1247.
[54] PAONE P, CANI PD. Mucus barrier, mucin, and Intestinal microbiome: Expected sticky partners [J]. Intestinal, 2020, 69: 2232‒2243.
[55] SLIVE ZM, BLIKSLAGER AT. The integral role of tight junction proteins in the repair of injured intestinal epithelium [J]. Int J Mol Sci, 2020, 21(3): 972.
[56] SICARD JF, BIHAN G, VOGELEER P, et al. Interaction of intestinal bacteria with intestinal mucus components [J]. Front Cell Infect Microbiol, 2017, 7: 387.
[57] SUPINDA BUNYAVANICH M, BERIN C. Food allergy and the microbiome: Current understandings and future directions [J]. J Allergy Clin Immun, 2019, 144(6): 1468-1477.
[58] STUMPF F. Look at the stinky side of physiology: Transport of short-chain fatty acids [J]. Pflugers Arch, 2018, 470: 571‒598.
[59] WANG KP, CHENG F, PAN XL, et al. Investigation of the transport and absorption of Angelica sinensis polysaccharide through gastrointestinal tract both in vitro and in vivo [J]. Drug Deliv, 2017, 24(1): 1360‒1371.
[60] BAI JY, LI TT, ZHANG WH, et al. Systematic assessment of oat β-glucan catabolism during in vitro digestion and fermentation [J]. Food Chem, 2021, 348: 8308‒8146.
[61] KOH A, VADDER FD, PETIA KD, et al. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites [J]. Cell, 2016, 165(6): 1332‒1345.
[62] ZHANG DD, LIU J, CHNE G, et al.Interactions between polysaccharides and gut microbiota: A metabolomic and microbial review [J]. Food Res Int, 2022, 160: 0963‒9969.
[63] CHONG J, MA JD, ZHANG JW, et al. Research progress of intestinal immune regulation by SCFAs [J]. Life Sci, 2023, 35(5): 663‒670.
[64] CHRIST S, DABEK A, VOITALA M, et al. The prominent role of butyrate relative to beta-hydroxybutyrate as a histone deacetylase inhibitor, transcriptional regulator and anti-inflammatory molecule [J]. Sci Rep, 2019, 9(1): 742.
[65] MARKUS W, KAUTENBUEGER T, DAUMANN H, et al. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon [J]. J Nutr Biochem, 2008, 19(9): 587‒593.
[66] KNETHEN VA, HEINICKE U, WEIGERT A, et al. Histone deacetylation inhibitors act as regulators of regulatory T cells [J]. Mol Sci, 2020, 21(7) : 2356.
[67] TAN JK, MACIA L, MACKAY CR. Dietary fiber and SCFAs in the regulation of mucosal immunity [J]. J Allergy Clin Immunol, 2023, 151(2): 361‒370.
[68] NADEEM A, SIDDIQUI N, ALHARBI NO, et al. Acute glutathione depletion leads to enhancement of airway reactivity and inflammation via p38MAPK-iNOS pathway in allergic mice [J]. Int Immunopharmacol, 2014, 22 (1): 222‒229.
[69] LOVING CL, SHAWN MDB, BRADLEY LB, et al. Effect of dietary β-glucan on intestinal microbial diversity and Salmonella vaccine immunogenicity and efficacy in pigs [J]. Vet Microbiol, 2023, 278:
1378‒1135.
[70] ALJEWICZ M, NALEPA B, CIESIELSKI S. The influence of different types of β-glucans on the gut microbiota of rats fed milk gels [J]. J Funct Foods, 2022, 89: 1756‒4646.
[71] COSTANZO M, PAULISN, BIASUCCI G. Butyrate: A link between early life nutrition and gut microbiome in the development of food allergy [J]. Life, 2021, 11(5): 384.
[72] WANG CC, WU H, LIN FH, et al. Sodium butyrate enhances intestinal integrity, inhibits mast cell activation, inflammatory mediator production and JNK signaling pathway in weaned pigs [J]. Innate Immun, 2017, 24: 40‒46.
[73] RORIE JW, KEOWN NM. Understanding the physics of functional fibers in the gastrointestinal tract: An evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber [J]. J Acad Nutr Diet, 2017, 117(2): 251‒264.
[74] SIMA P, VANNUCCI L, VETVICKA V. β-glucans and cholesterol (Review) [J]. Mol Med, 2018, 41: 1799‒1808.
[75] LIU B, YANG T, LUO Y, et al. Oat β-glucan inhibits adipogenesis and hepatic steatosis in high fat diet-induced hyperlipidemic mice via AMPK signaling [J]. J Funct Foods, 2018, 41: 77‒82.
[76] MO XX, SUN YH, LIANG XL, et al. Insoluble yeast β-glucan attenuates high-fat diet-induced obesity by regulating gut microbiota and its metabolites [J]. Carbohyd Polym, 2022, 281: 8144‒8617.
[77] SEIICHIRO A, YASUNORI I, NORIKO K, et al. Effects of high β-glucan barley on visceral fat obesity in Japanese individuals: A randomized, double-blind study [J]. Nutrition, 2017, 42: 1‒6.
[78] CHAKRABORTY S, RAJESWARI VD. Biomedical aspects of beta- glucan on glucose metabolism and its role on primary gene PIK3R1 [J]. J Funct Foods, 2022, 99: 1756‒4646.
[79] GUO HQ, WU HL, KONG XQ, et al. Oat β-glucan ameliorates diabetes in high fat diet and streptozotocin-induced mice by regulating metabolites [J]. JNutr Biochem, 2023, 113: 2955‒2863.
[80] WU LJ, ZHAO J, ZHANG XN, et al. Antitumor effect of soluble β-glucan as an immune stimulant [J]. Int J Biol Macromol, 2021, 179: 116‒124.
[81] CHOROMANSKA A, KULBACKA J, REMBIALKOWSKA N, et al. Anticancer properties of low molecular weight oat beta-glucan-An in vitro study [J]. Int J Biol Macromol, 2015, 80: 23‒28.
[82] XIE WY, SHAO F, DUAN XH, et al. Whole β-glucan particle attenuates AOM/DSS-induced colorectal tumorigenesis in mice via inhibition of intestinal inflammation [J]. Front Pharmacol, 2023. DOI: 10.3389/fphar. 2023.1017475
[83] ZHANG XR, LI TT, LIU SW, et al. β-glucan from Lentinus edodes inhibits breast cancer progression via the Nur77/HIF-1α axis [J]. Biosci Rep, 2020. DOI: 10.1042/BSR20201006


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






