Coenzyme Q 10 Preparation Technology and Its Application in Medicine
Coenzyme Q 10, also known as ubiquinone, is a fat-soluble quinone compound that is widely distributed in nature, mainly in yeast, plant leaves, and seeds, and in the cells of the heart, liver, and kidney of animals. It was first isolated from bovine heart muscle by Frederick in 1957.
Coenzyme Q 10 is an important member of the mitochondrial respiratory chain. It binds to the mitochondrial inner membrane and is involved in cellular oxidative phosphorylation and ATP production. Coenzyme Q 10 is a natural antioxidant of the cell itself and a stimulant of cellular metabolism. It is also associated with the reticuloendothelial system and can enhance immunity[1] . As the only coenzyme Q substance in the human body, abnormal metabolism of coenzyme Q 10 has been associated with many diseases in the human body and artificial supplementation of coenzyme Q10 can alleviate many diseases[2-3].
Studies over the years have shown that Coenzyme Q 10 is a good adjuvant for cardiac diseases, protecting the heart during cardiac surgery without any toxic or side effects. Since 1977, many international symposiums have been held to study its biological properties and clinical applications[4], and the evaluation of its efficacy in practical applications has been increasing day by day. Therefore, the development of coenzyme Q10 has a broad market prospect.

1.Physical And Chemical Properties of Coenzyme Q 10
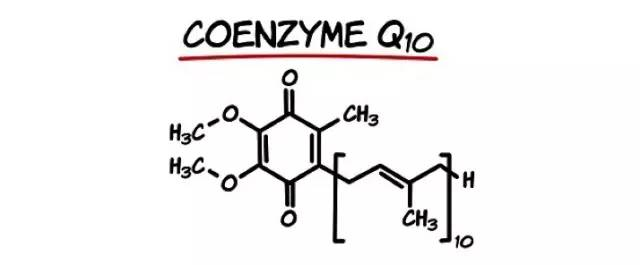
Coenzyme Q is a family of fat-soluble quinones, the chemical structure of which is shown in Figure 1. 10 is the number of isoprenoid units in the side chain of Coenzyme Q. Coenzyme Q is found in a wide range of animals, plants, and microorganisms in nature. Among the discovered substances, the five naturally occurring members of coenzyme Q, namely Q6, Q7, Q8, Q9, and Q10, have similar physical and chemical properties. This is because they share the same parent ring and differ only in the isoprenoid chain length of the side chains[5]. In addition, coenzyme Q13 was found in a mutant strain of Escherichia coli.
Fig.1 The Chemistry Structure of Coenzyme Q 10
Coenzyme Q 10 has the molecular formula C59 H90 O4 and the molecular mass is 863.36u. It is an orange-yellow crystal at room temperature with a melting point of 49°C. It is odorless and tasteless. It is odorless and tasteless. It decomposes easily into a reddish color under light and is stable to temperature and humidity. Because of its long isoprene-like side chain, it is soluble in chloroform, benzene, and carbon tetrachloride, soluble in acetone, petroleum ether, and ether, slightly soluble in ethanol, insoluble in water and methanol.
2.Coenzyme Q10 Preparation Method
There are three main methods for the preparation of coenzyme Q10, namely, plant and animal tissue extraction, microbial fermentation, and chemical synthesis. In the past, there was also a plant cell culture method, the use of tobacco cell culture technology to prepare coenzyme Q10, which is one of the products of the development of plant cell culture technology, from the tobacco culture cells to obtain coenzyme Q10 crystals. However, the production cost of this method is high and it is not suitable for industrial production, so the relevant reports of this method are rare in recent years[6] .
2.1 Chemical Synthesis
Japan was the first country to develop Coenzyme Q10, which was produced by chemical synthesis by Nisshin Corporation. In the chemical synthesis of Coenzyme Qn, 2,3-dimethoxy-5-methyl 1,4-benzoquinone, also known as Coenzyme Q10, is the necessary intermediate. The main method for its synthesis is the oxidation of 3, 4, 5-trimethoxytoluene. There are two main methods reported in the literature: one is the ammonium persulfate oxidation method, due to the use of sulfuric acid medium, the reaction solution is very dark in color, extraction and separation is difficult, the purity of the product is low, and the dosage of acetic acid is very large; the other is the hydrogen peroxide oxidation method, which adopts heteropolymeric acid as the catalyst and formic acid as the solvent, and oxygenates the reaction solution with hydrogen peroxide of 500mL/L, and then analyzes the reaction solution by column analysis, with a yield of up to 85% [7]. However, because the peroxyformic acid generated in the reaction is extremely unstable, the reaction is rapid, the reaction temperature is difficult to control, and a large number of by-products are generated, which need to be separated by column chromatography, and it is not easy to realize industrial production.

2.2 Microbial Fermentation
Currently, the most promising method is microbial fermentation, and the key technology of this method is the production capacity of coenzyme Q10-producing bacteria and how to extract coenzyme Q10 from the fermented bacteria. Many microorganisms can be used to produce coenzyme Q10 (Table 1) [8-9]. As can be seen from the table, the distribution of coenzyme Q10 is heterogeneous among microorganisms, with the absence of coenzyme Q10 in cells that do not require aerobic respiration, such as Gram-positive bacteria, and the presence of high concentrations of coenzyme Q10 in Gram-negative bacteria. Since coenzyme Q10 is composed of an aromatic ring and an isopentenyl side chain, the aromatic ring must first be synthesized in the microorganism. There are two routes of synthesis, one is from mangiferolic acid via p-hydroxybenzoic acid, and the other is from acetic acid-acetone.
Table 1 Content of Coenzyme Q10 from Some Microbe
Name of Microbe | Contents/(nmol . mL—1) |
Ncurospora Crassa | 61 |
Rhodopscudonomas Palustris | 190 |
Agrobacteriumumefacins | 290 |
Cryptocoeeus Laurentii | 420 |
Aspergillus fumigatus | 500 |
Sporobolomyces Roseus | 463 |
Pseudomonas Denitrificans | 1 200 |
In 1977, Japan realized the first fermentation method for the production of Coenzyme Q10, but the efficiency was low and the production cost was high. Nowadays, with the rapid development of bioengineering, especially genetic engineering, the production process of Coenzyme Q10 has made great progress. For example, R. spheroides co22-11 (FERM-p6008) was cultured in a sterile medium containing 30 g/L molasses, 30 g/L glucose, 20 g/L yeast paste, 5 g/L ammonium sulfate and 20 g/L calcium carbonate at 30 ℃ for 3d~7d, and the final fermentation broth contained 300mg/L of coenzyme Q10, which is double the amount of fermentation of the common producer. The fermentation broth contained 300 mg/L of Coenzyme Q10, which was 2-5 times the fermentation volume of the common producer bacteria. Therefore, the insertion of the target gene is more favorable for the accumulation of Coenzyme Q10 in the bacteria and the enhancement of the fermentation efficiency[10].

In recent years, the research on microbial fermentation and extraction has been progressing in China, mainly in the Laboratory of Pharmaceutical Engineering of the University of Chemical Technology of Beijing and in the Graduate School of the Chinese Academy of Sciences. However, due to the problem of bacterial strain, the content of coenzyme Q10 in the bacterial liquid is very low. After screening, a strain of coenzyme Q10 was obtained from the Beijing Institute of Chemical Technology, and after optimization, the output reached 160mg/L fermentation liquid, but it is only 1/5~1/2 of foreign products, therefore, it is difficult to realize industrial production in terms of cost.
2.3 Plant and Animal Tissue Extraction
Currently, plant and animal tissues are mostly used in China, and Coenzyme Q10 is found in large quantities in some plant and animal tissues (Table 2). The main extraction methods are:
Table 2 Contents of Coenzyme Q10 in the Tissues of Animal and Plant
Name of Material | Contents/(nmol . mL—1) |
Tecoma Stans | 19 |
Zeamays | 26 |
Tamarindus Indica | 45 |
Spinacia Oleracea | 49 |
Arummaculatum | 350 |
Pig Liver | 35 |
Cattle Liver | 40 |
Cattle Heart | 85 |
Pig Heart | 98 |
Goat Heart | 200 |

(1) Saponification Method
①Alkali Saponification Method
The raw material cells containing Coenzyme Q10 were crushed in acidic aqueous solution, heated at 100 ℃ for 3 h, added sodium hydroxide, and saponified at 100 ℃ for 1 h. The saponified solution was extracted with ethane mixed with isopropanol. The saponified solution was extracted with ethane mixed with isopropanol, the extract was concentrated, cholesterol was removed from the precipitate by cold dialysis, and a silica gel chromatography column was used to collect the Coenzyme Q10 solution, concentrate it, and crystallize it with anhydrous ethanol to obtain Coenzyme Q10. The production cost of Coenzyme Q10 is reduced by direct saponification using acid-crushing cells.
② Alcohol Alkali Saponification Method
Weigh a certain amount of tissue material, add a certain proportion of pyrogallic gallic acid, stir well, and then slowly add 100 g/L sodium hydroxide - ethanol solution stirring, then the tissue material into a black paste, heat reflux in a water bath for 30 min. Quickly cool to room temperature, add a certain amount of petroleum ether, and repeat extraction, the extract was washed with water to neutral. Then remove the water with anhydrous sodium sulfate, the purpose of removing water is to make coenzyme Q10 and hydrocarbon solvents better miscible, to facilitate the subsequent processing. Concentrate to a small volume, chromatography with silica gel adsorption column, wash with petroleum ether to remove impurities, then eluted with a mixture of ethyl ether and petroleum ether, collect in segments, distilled to remove the solvent, and obtain a yellow oil. Crystallize with anhydrous ethanol to obtain orange-yellow crystals, i.e. Coenzyme Q10. This method is a classic extraction method, although simple but costly.
③Adsorption Chromatography
The raw material containing Coenzyme Q10 was extracted with a low concentration of ethanol solution for 3 h at 73 ℃. The extract was adsorbed by macroporous resin, washed with a low concentration of ethanol, eluted with n-ethane, and concentrated, and the ethanol was removed to obtain the oily substance containing Coenzyme Q10. According to a Japanese report, the purity of Coenzyme Q10 was 99.4% by chromatography on an Amberlite XAD column, followed by a Hipores Hp-20 column, elution with a 9:1 acetone aqueous solution, concentration, and crystallization in ethanol.
3.Medical Applications of Coenzyme Q10
3.1 Clinical Applications of Coenzyme Q10 in Cardiovascular Diseases
Cardiovascular disease is the main application of coenzyme Q10, Folkers K et al[11] found that the myocardium of patients with cardiac disease has reduced coenzyme Q10 activity, arterial stenosis, atresia, and other deficiencies of coenzyme Q10. After oral administration of Coenzyme Q10, it is absorbed through the lymphatics enters the mitochondria of the cells, and then acts directly on the ischemic heart, which can improve the utilization of oxygen.
Coenzyme Q10 is effective in ischemic heart disease, hypertension, and rheumatic congestive heart failure symptoms (edema, pulmonary congestion, hepatomegaly, and angina)[12]; the addition of Coenzyme Q10 to conventional antimyocardial ischemic drug therapy can significantly reduce the number of episodes of angina, reduce the dosage of nitrate, and improve the rate of conversion to ischemic S-T and T-wave alterations in exercise tolerance[13]. Coenzyme Q10 is effective in the treatment of angina pectoris. Coenzyme Q10 is also effective in the treatment of hypertension, and its antihypertensive effect is relatively mild without adverse side effects.
The possible explanation for the lowering of blood pressure by coenzyme Q10 is that it acts as a cardiovascular antioxidant and acts directly on the vascular wall to eliminate peroxides or inhibit their synthesis in the vasculature. By improving the shuttling efficiency of high-energy electron transport from the cytoplasm to the mitochondrial respiratory chain, coenzyme Q10 reduces cytoplasmic levels of reduced coenzyme I (NADH) and eliminates the reduced potential that drives peroxide synthesis in the endothelium and vascular smooth muscle.14 Coenzyme Q10 is also known to lower blood pressure in patients with hypertension.
Certain antihypertensive drugs can exacerbate the deficiency of coenzyme Q10 in the heart of hypertensive patients, and coadministration of coenzyme Q10 with antihypertensive drugs not only enhances the antihypertensive effect but also prevents the side effects of the drugs. Coenzyme Q10 has a protective effect on the myocardium in congestive heart failure, cardiomyopathy, coronary artery disease, acute myocardial infarction, and other cardiac dysfunctions[15]. Coenzyme Q10 can lower lipoprotein and plasma insulin, inhibit cholesterol oxidation, and prevent arterial atherosclerosis. In conclusion, the clinical efficacy of coenzyme Q10 in cardiovascular disease is mainly due to its improvement in myocardial energy production, its antioxidant activity, and its biofilm stabilizing properties[16].
3.2 Other Medical Applications of Coenzyme Q10
Studies have shown that Coenzyme Q10 has anti-tumor effects in experimental animals. Geng Ailian et al[17] reported that dietary supplementation of coenzyme Q10 could significantly reduce the morbidity and mortality of ascites in broiler chickens, and reduce the osmotic fragility of erythrocytes in broiler chickens (EOF) (p≤0.05). The mitochondria of human liver, gastric, and intestinal cancer cells are significantly deficient in coenzyme Q10, and the hemoglobin cells of patients with advanced cancers are also deficient in coenzyme Q10 by about 50%, so the administration of coenzyme Q10 to patients with certain tumors can stabilize their conditions and prolong their lives. Coenzyme Q10 has also been used in the protection of pneumonia, hepatitis B, and kidney disease[18].
Coenzyme Q10 is effective in the treatment of scurvy, diabetes mellitus, progressive myasthenia gravis, duodenal and gastric ulcers, necrotizing periodontitis, and viral hepatitis. It is also effective in treating round alopecia, emphysema, and hearing impairment. In recent years, Coenzyme Q10 has also been found to be effective in the treatment of AIDS. Coenzyme Q10 has also shown promising results in anti-aging. Therefore, in addition to playing an important therapeutic role in some alternative treatments, CoQ10 is also an adjunctive medicine with a wide range of applications.
References:
[1] QIAN Xue , WANG Zuqiao , KORAN Ping . Pharmacology and application of coenzyme Q10 [J]. Food and Drugs, 2006, 8(1): 16-19.
[2] LIU Xiangning, FENG Zhiqiang, LI Xiaoling. Treatment of recurrent aphthous ulcer with coenzyme Q10 in combination with gold-vitamin 21 in 59 cases[J]. Journal of Jinan University (Medical Edition), 2005, 26(2): 267-268.
[3] Shin L. Coenzyme Q10 slows the progression of Parkinson's disease[J]. First Point of View, 2002, (22): 9.
(22): 9.
[4] Folkers K. Biomedical and clinical aspects of coenzyme Q[M]. Elserier Holland , 1977: 316.
[5] Wu PF , Lu ML , Tao WY . Separation and determination of coenzyme Q10 in Mycobacterium smegmatis by saponification[J]. Biotechnology, 2006, (1):43-45.
[6] HUANG Wei, XU Jianzhong, FENG Xiaoliang. Research on Coenzyme Q10[J]. Henan Chemical Industry, 2003, (2): 12-14.
[7] chida A S, parimi V S, chandrasekharam M , et al. Synthesis of 2, 3-dimethoxy-5-methyl-1, 4-benzoquinone: a key fragment in coenzyme-Q series[J]. Synthetic communications, 2001, 31(5): 657-660.
[8] Shen T. Biochemistry[M]. Beijing: People's Education Publishing House, 1990.
[9] Lenaz G. coenzyme Q biochemistry [M]. New York: A Wiley Interscience publication, 1984: 33-47.
[10] Ouyang PK, Hu YH. Production and application of coenzyme Q10 [J]. Chemical Engineering Progress, 1994, (4): 9-11.
[11] Folkers K. Heart failure is a dominant deficiency of Q10[J]. Clin Investing, 1993, 71:851.
[12] Liu WL. Observations on the efficacy of coenzyme Q10 in the treatment of angina pectoris in coronary heart disease[J]. Chinese Modern Medical Science and Technology, 2003, 3(2):25.
[13] Greenberg S M, Frishman W H. Coenzyme Q10 a new drug for cardiovascular disease[J]. J Clin Pharmacol, 1990, 30: 596.
[14] Mocarty M F. Coenzyme overseers hypertension: Does coQ de- crease endothelial superoxide generation[J]. Med Hypotheses, 1999, 53(4): 300.
[15] Ding Yanyan, Zhang Weixue, Lu Fanghao. Protective effect of coenzyme Q10 on isoproterenol-induced myocardial ischemia[J]. Journal of Harbin Medical University, 2006, 40(1): 40-41.
[16] Colucci W S, Wright R F, Braunwald E. New positive inotropic agents in the treatment of congestive heart failure, mechanism of action and recent clinical developments[J]. N Endl J Med , 1986, 314: 349.
[17] Geng Ai-lian, Lopsided Yu-ming, Yuan Jian-min, et al. Effects of coenzyme Q10 on the performance of broiler chicks and its sensitivity to ascites[J]. Journal of Animal Husbandry and Veterinary Medicine, 2005, 36(1): 16-20.
[18] Xue H. Coenzyme Q10 as an adjuvant for the treatment of asthmatic pneumonia in 50 cases [J]. Railway Medicine, 1999, 27(2): 112-114.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese