Allulose What Is It Made From?
With the increasing prevalence of obesity and diabetes, “healthy eating and low-sugar living” has become a popular trend in today's society. In 2019, the International Diabetes Federation (IDF) released a report stating that 9.3% of adults aged 20 to 79 worldwide have diabetes, which means 463 million people have diabetes. Medical expenses for diabetes account for 10% of global health expenditure (approximately 760 billion US dollars) [1~2]. In response to the daily sweet cravings of these patients and the obese, there is an urgent need to develop sweeteners that are similar to sucrose in sweetness but do not cause blood sugar to rise.
Allulose can meet these needs due to its sweetness similar to sucrose, extremely low calorie content and its special physiological effects. In recent years, demand in foreign markets has been on the rise. Domestic universities, research institutes and enzyme preparation companies have rapidly developed research on allulose in recent years, and the number of related papers published has increased year by year[3].
1. Physicochemical properties and physiological functions of D-allulose
D-allulose, also known as D-ribo-2-hexulose, is a diastereoisomer of D-fructose at the C-3 position, and can be prepared by adding diastereoisomerase to D-fructose. It was originally isolated from psicofuranine and therefore named D-psicose[4]. In 2014, the International Rare Sugar Conference held in Japan officially corrected the conventional name of D-psicose from D-psicose to D-allulose[5~6].
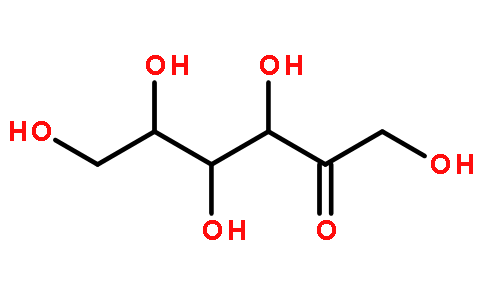
1. 1 Physical and chemical properties of D-allulose
D-Allulose is a typical rare hexose. It is a white crystalline powder and an isomer of glucose and fructose. Its molecular weight is 180.16 and its molecular formula is C6H12O6. It is very soluble in water. at room temperature, 100g can dissolve 291g of allulose in water[7]; melting point is 109℃, stable under normal temperature and pressure. Because of its low melting point, it is not suitable for spray drying to produce powdered products. The sweetness of D-allulose is 70% that of sucrose [8], and its sweetness is mild. Eating the same amount of allulose will only generate 0.3% of the calories of sucrose [9].

1.2 Physiological functions of D-allulose
1. 2. 1 Neuroprotective effect
Japanese scientists Takata et al. [10] found that 50 mmol/L D-allulose can protect nerves by increasing the intracellular glutathione level and inhibiting the apoptosis of catecholamine PC12 cells induced by the neurotoxin 6-hydroxydopamine. Adding D-allulose to the daily diet can effectively reduce the incidence of Parkinson's disease.
1. 2. 2 Lowering blood sugar
Relevant studies have shown that after oral intake of D-allulose, the activity of glucose glycosidase and α-amylase in the intestine can be effectively inhibited by D-allulose[11], thereby significantly lowering the blood sugar level after a meal. In a small clinical trial, healthy subjects who consumed 75 g of maltodextrin and a dose of D-allulose of 5 g or more had lower plasma glucose and insulin concentrations [12].
1. 2. 3. Lipid-lowering and weight-reducing effects
Ochiai et al. [13] showed that D-allulose feeding significantly increased lipase activity in rats. Matsuo et al. [14] confirmed experimentally that after rats were fed D-allulose for 28 days, the activity of their liver lipogenic enzyme was significantly reduced, and the abdominal adipose tissue was significantly lower than that of rats fed D-fructose [8]. Studies have confirmed that D-allulose can promote cholesterol reverse transcription and lower high-density cholesterol, which has the effect of preventing atherosclerosis [15]. In addition, numerous studies have shown that D-allulose has a strong ability to scavenge reactive oxygen species (ROS) [16].
2 Preparation of D-allulose
D-allulose is rarely found in nature, only in small amounts in figs, sugar cane molasses, wheat and rat-thorn plants, so it is not suitable for extraction from plants. At present, the main methods for preparing D-allulose are chemical synthesis and biological enzyme conversion.
2. 1 Chemical synthesis method
A relatively typical chemical synthesis method for D-allulose is to use glucose as the raw material, molybdate as the catalyst, and synthesize at 80~120℃. Although the maximum conversion rate of this method can reach more than 40%, the ash content of the product is too high to meet food requirements. In addition, the conductivity of the reaction solution is as high as 10,000–20,000 μs/cm [17], which requires electrodialysis and purification and desalination with multiple anion and cation exchange resins, resulting in an increased sewage treatment load. Fang Zhijie et al. [18] developed a method for the chemical synthesis of allose from glycerolide. The use of chemical solvents such as toluene and acetonitrile in the process is not only harmful to the human body, but also cumbersome. Other methods such as catalytic hydrogenation and Ferrier rearrangement[19] also have problems such as low conversion efficiency and serious environmental pollution, and are not suitable for large-scale industrial production.
2. 2 Biological enzyme conversion method
Research on D-allulose biocatalysis began relatively early abroad. Ken Izumori of the Kagawa University Rare Sugar Research Center in Japan proposed a set of rare sugar conversion strategies based on years of research into rare sugar bioconversion, which is known as the “Izumoring” method [20]. According to the Izumoring rare sugar conversion strategy, there are currently two main methods in bioconversion that can achieve the production of D-allulose. One is the use of oxidoreductases to generate D-allo-keto-sugars from allo-initol (alloin), taro-initol and D-galacto-initol. Due to the high cost of the substrates in this method, it is not commercially viable from a cost perspective [21-22]. Another method is the isomerization of D-fructose to D-allulose by an isomerase. In 1993, Izumori et al. [23] discovered an enzyme from Pseudomonas cichorii ST-24 that can catalyze the isomerization of C3 of hexose. Since its optimal substrate is tagatose, it was named D-tagatose 3-epimerase (DTE enzyme, hereinafter referred to as DTE).
In 2006, Kimh et al. [24] from South Korea isolated D-allulose 3-epimerase (D-Psicose 3-Epimerase, DPE enzyme, hereinafter referred to as DPE) from Agrobacterium tumefaciens, and its optimal substrate is D-allulose. DPE exhibits higher D-fructose isomerization activity than DTE[8]. Compared with foreign research, domestic research on D-allulose started relatively late. It was not until 2008 that Jiang Bo's team at Jiangnan University[25] selected a DTE from Rhodobacter sphaeroides named SK011 from 30 mud and water samples. After shake flask culture, whole-cell transformation was carried out, and the yield was only 6.54%. Since 2010, research on DPE/DTE has developed rapidly in China, with five master's and doctoral dissertations published in 2021 alone [26–30].
Among them, Wu Jing's team at Jiangnan University has continuously optimized the molecular modification and fermentation culture conditions of the DPE gene derived from Clostridium cellulolyticum H10, and increased the fermentation enzyme activity of a 3L tank to 4567μ/mL[26]. At present, there are more than 400 DPE enzyme genes annotated in the US National Center for Biotechnology Information, and more than 20 have been clearly reported in the literature. The recombinant enzyme has a fructose conversion rate of about 30%. Adding borate can increase the conversion rate, and the borate in the allulose borate complex can be easily removed using Amberite IRA-743 and Dowex 50 resins [31].
3. Development direction of suitable D-allulose industrial enzymes
Although there have been significant developments in D-allulose in recent years, most of them have focused on gene mining and strain construction, and there have been relatively few reports on the isolation and purification of the product [32]. There are still some deficiencies in the research and development of the product and its effective combination with industrialization. Since D-allulose is mainly used in food, the following describes the direction of industrialization needs in combination with the requirements of food laws and regulations and the problems found in the industrialization process.
3.1 Selection of expression strains of recombinant bacteria
Due to the limitations of natural enzymes, such as instability, narrow substrate spectrum and low catalytic efficiency, they are not suitable for direct application in production. It is necessary to modify the gene encoding the target protein at the molecular level, and then screen for mutants with significantly improved properties[26]. Taking food safety and compliance as well as industrialization into consideration, the choice of recombinant bacteria should be based on food-grade microorganisms, non-pathogenic, not easily infected by phages, and with the ability to secrete proteins efficiently. Bacillus subtilis is an aerobic Gram-positive bacterium that can form spores and has a cell wall that does not contain endotoxins. It is the earliest species in the genus Bacillus to be used as a genetic engineering host. As a microorganism recognized as safe by the FDA, Bacillus subtilis has long been used in the preparation of fermented foods. Recombinant Bacillus subtilis has the advantages of simple and rapid culture, good fermentation basis and production technology, and is an ideal expression host for industrial enzymes[33] .
3. 2 The direction of DPE enzyme modification
Although the research direction in recent years has taken into account the possibility of industrialization to a certain extent, it is mostly focused on improving the thermal stability of the DPE enzyme. The following describes the requirements for industrial enzymes from an industrialization perspective.
3.2.1 Optimal pH of the enzyme
The optimum pH for enzymes is preferably weak acidity, and the wider the range, the more conducive to industrialization. According to the rare sugar conversion strategy, the one-step enzymatic production of D-allulose uses relatively inexpensive fructose as a substrate and is catalyzed by DPE enzyme to produce allulose. However, the production of allulose from fructose by DPE is a reversible reaction, and enzyme treatment is required after the reaction is complete. The substrates fructose and allulose are more likely to undergo the Maillard reaction under conditions of high temperature, alkalinity and the presence of protein, which increases the cost of decolorization with activated carbon for subsequent sugar refining. The industrial choice of the most suitable pH value is weakly acidic DPE, which is more suitable. The effect of different pH values on the transmittance and absorbance of the feed solution is shown in Table 1.
As can be seen from Table 1, when the initial pH of the reaction is weakly acidic, the transmittance and color value of the reaction solution are better than those of the solution with an initial pH of neutrality.
In the aforementioned literature, DPE enzyme catalyzes the preparation of allose from fructose. The dissolution of fructose must be maintained with a buffered salt solution to stabilize the pH value, which will lead to an increase in the conductivity of the solution, causing the subsequent anion and cation exchange processes to have a problem with the decline in the number of times the material can be passed through the resin. Industrial production is not easy to adopt, and it is sufficient to dissolve it in deionized water. Yuan Tangguo et al. [3] and Li Xiaobo et al. [21] confirmed this through experiments.
3. 2. 2 Metal ion selectivity
Most DPE enzymes are metal-dependent enzymes. Even if some are not metal-dependent, the presence of certain metal ions can greatly promote the reaction rate [21]. From the perspective of food safety, it is advisable to choose metal ions such as Mg2+ and Mn2+ as food additives for the industrial preparation of enzymes [34~35].
3.2.3 Optimum temperature of the enzyme
Studies have shown that the higher the reaction temperature, the shorter the half-life of the enzyme, but at the same time the reaction rate is faster and the production cycle is shortened. From the perspective of industrial production, the optimum temperature of the enzyme is not the higher the better. The higher the temperature, the greater the steam consumption, and the Maillard reaction is likely to occur. A balance needs to be found between the two, and generally controlled between 40 and 60 °C, taking into account the production of other functional sugars. The higher the optimum temperature of the enzyme, the more conducive it is to improving the enzyme activity recovery rate during the extraction process of crude enzyme solution.
3. 3 Substrate concentration suitable for industrial application
The effect of substrate concentration on enzyme conversion is shown in Table 2.
As can be seen from Table 2, the lower the concentration of the fructose solution, the faster the conversion in the early stage of the reaction with the same amount of added enzyme (to the dry basis of the reaction substrate). However, if the concentration is too low, the amount of allulose produced per unit time will be too high. With the industrialization of high-concentration decolorization and high-concentration ion exchange, the conversion of low-concentration enzymes to subsequent concentration and other processes has increased the workload. Therefore, in industrial production, a concentration of about (400-500) g/L can also achieve a high conversion rate, and can reduce water and energy consumption and reduce carbon emissions.
3. 4 Preparation and problems with enzyme preparations
The reaction rate of enzyme solution is much higher than that of whole cells. Under the same conditions of adding enzyme, when the concentration of fructose solution is about 300 g/L, the reaction rate of crude enzyme solution is about 5 times that of whole cells. DPE enzyme is an intracellular enzyme, and the process of preparing the enzyme solution involves centrifugation, washing and homogenization. Although crude or pure enzyme solutions can be prepared at low temperatures at the laboratory level, it is difficult to maintain a low temperature during centrifugation and homogenization during industrial production. In particular, the loss of enzyme activity during the homogenization process is relatively high, resulting in high enzyme production costs.
4 Prospects
D Allulose has now been approved by regulations in 13 countries, including Japan, South Korea, Canada, Mexico, Singapore and Australia. In April 2019, the FDA even announced that D-allulose would be excluded from the “added sugar” and “total sugar” labels[2], which means that the amount of D-allulose added to food will no longer be restricted in terms of the amount added. However, due to the relatively high price of allulose at present, sales are not yet very large. With the deepening of research and the gradual maturity of the food-grade host recombinant expression of the key DPE enzyme technology for D-allulose, the conversion rate of D-allulose will also continue to improve. Enterprises will continue to improve problems identified during industrialization and enzyme preparation enterprises will launch finished enzymes.
The preparation cost of allulose will decrease significantly and product product quality will be further improved. In August 2021, China's National Health Commission has accepted the application for D-allulose as a new food ingredient. Toxicological experiments are currently underway, and it is expected that the use of allulose as a sweetener will be approved in the second half of 2023 or 2024. Since D-allulose is hardly metabolized after passing through the intestines, it does not provide energy, and it has unique physiological effects such as effectively reducing postprandial blood glucose, controlling body weight, and reducing fat accumulation, its application fields will become wider and wider. In the next few years, the market space and capacity for D-allulose at home and abroad will continue to expand, and the market prospects are good.
Reference:
[1] International Diabetes Federation diabetes atlas ninth edition 2019[R] .IDF , 2019.
[2] Guo Yuanheng, Wang Jing, Wang Xiaoyan, et al. Research and industrialization progress of biosynthesis of D-allulose in China [J]. Modern Food, 2020(6): 34-40.
[3] Yuan Tanguo. Heterologous expression of D-allulose isomerase and efficient conversion of D-allulose [D]. Dalian: Dalian University of Technology, 2021.
[4] Eble T E , Hoeksema H , Boyack G A , et al .Psicofuranine. Ⅰ .Dis- covery, isolation , and p rop erties[J] .Antibiotics & Chemotherapy (Northfield , Ill. ) ,1959 , 9(7) :419-420.
[5] Park C S , Kim T , Hong S H , et al . D-Allulose p roduction from D-Fructose by p ermeabilized recombinant cells of Corynebacte- rium glutamicum cells exp ressing D-allulose 3-epimerase fla- vonifractor plauti[J] .Plos One , 2016 , 11(7) : 160044.
[6] Liu Menglu, Yuan Weitao, Li Ning, et al. Research progress of the functional sweetener D-allulose [J]. China Food Additives, 2022, 33(1): 21-25.
[7] Kimura T , Kanasaki A , Hayashi N , et al . D-Allulose enhances postprandial fat oxidation in healthy humans [J] . Nutrition , 2017 , 16 :43-44.
[8] Chung M Y , Oh D K , Lee K W.Hypoglycemic health benefits of D-p sicose[J] .Journal of Agricultural and Food Chemistry, 2012 , 60(4) :863-869.
[9] Matsuo T , Suzuki H , Hashiguchi M , et al . D-p sicose is a rare sugar that p rovides no energy to growing rats [J] .Journal of Nu- tritionalScience & Vitaminology, 2002 , 48(1) :77-80.
[10] Takata M K , Yamaguchia F , Nakanosea K , et al .Neuropro-tec- tive effect of D-Psicose on 6-hydroxydopamine-induced apopto- sis in rat pheochromocytoma (PC12) cells[J] .Journal of Biosci- ence and Bioengineering, 2005 , 100:511-516.
[11] Matsuo T , Izumori K. D-p sicose inhibits intestinalα-glucosidase and supp resses the glycemic response after ingestion of carbo- hydrates in rats [J] .Journal of Clinical Biochemistry & Nutri- tion , 2014 , 54(3) :219.
[12] Iida T , Kishimoto Y , Yoshukawa Y , et al .Acute D-p sicose ad- ministration decreases the glycemic responses to an oral malto- dextrin tolerance test in normal adults[J] .Journal of Nutrition- al Science and Vitaminology, 2008 , 54(6) :511-514.
[13] Ochiai M , Onishi K , Yamada T , et al . D-Psicose increases ener- gy exp enditure and decreases body fat accumulation in rats fed a high-sucrose diet[J] . International Journal of Food Sciences and Nutrition , 2014 , 65(2) :245-250.
[14] Matsuo T , Baba Y , Hashiguchi M , etal.Dietary D-p sicose , a C- 3epimer of D-fructose , suppresses the activity of hepatic lipo- genic enzymes in rats [J] .Asia Pacifific Journal of Clinical Nu- trition , 2001 , 10(3) :233-237.
[15] Kanasaki A , Iida T , Murao K , etal . D-Allulose enhances uptake of HDL-cholesterol into rat's p rimary hepatocyte via SR-B1[J] . Cytotechnology, 2020 , 72(2) :295-301.
[16] Suna S , Yamaguchi F , Kimura S , et al .Preventive effect of D- p sicose , one of rare ketohexoses , on di-(2-ethylhexyl) phthalate (DEHP)-induced testicular injury in rat [J] . Toxicology Let- ters , 2007 , 173(2) : 107-117.
[17] Wang Chengfu, Fang Chunlei, Du Ruifeng, et al. A method for preparing allulose and its application [P]. CN104447888A, 2015-03-25.
[18] Fang Zhijie, Li Song, Cheng Jie, et al. A method for synthesizing rare hexopyranose and heptopyranose starting from sugar acid lactone [P]. CN101817851A , 2010-09-01.
[19] Doner L W.Isomerization of d-fructose by base:Liquid chroma-tographic evaluation and the isolation of d-p sicose[J] .Carbohy- drate Research , 1979 , 70(2) :209-216.
[20] Mu Wanmeng, Zhang Tao, Jiang Bo, et al. Bioconversion production strategy of rare sugars: Izumoring method [J]. Chinese Journal of Bioengineering, 2007, 27(7): 129-136.
[21] Li Xiaobo. Expression of D-allulose 3-epimerase and its immobilization for the conversion of D-allulose [D]. Tianjin: Tianjin University of Science and Technology, 2013.
[22] Izumori K , Yamakita M , Tsumura T , et al .Production of d-p si- cose from d-talitol , d-tagatose or d-galactitol b yAlcaligenes sp . 701B[J] .J Ferment Bioeng, 1990 , 70(1) :26-29.
[23] Izumori K , Khana R , Okaya H , et al . A new enzyme , D- ketohexose 3-Epimerase , from Pseudomonas sp . ST-24 [J] . Bioscience Biotechnology and Biochemistry, 1993 , 57(6) : 1037- 1039.
[24] Kimh J , Hyune K , Kimy S , et al . Characterization of an Agrobacterium tumefaciens D-p sicose 3-Epimerase that con- verts D-fructose to D-p sicose[J] . Applied and Environmental Microbiology, 2006 , 72(2) :981-985.
[25] Zhang Longtao, Mu Wanmeng, Jiang Bo, et al. Screening of clostridia for the bioconversion of D-allulose [J]. Food and Fermentation Industry, 2008(9): 40-43.
[26] Wang Yifan. Molecular modification, expression optimization and stability research of D-allulose 3-epimerase from Clostridium cellulolyticum H10 [D]. Wuxi: Jiangnan University, 2021.
[27] Bu Yifan. Optimization of the production of D-allulose 3-epimerase and its immobilization with cells [D]. Wuxi: Jiangnan University, 2021.
[28] Chen Jiajun. Identification of the properties of D-allulose 3-epimerase and research on the modification of thermal stability [D]. Wuxi: Jiangnan University, 2021.
[29] Feng Linxue. Study on the synthesis of D-allulose by multi-enzyme catalysis of D-fructose [D]. Wuxi: Jiangnan University, 2021.
[30] Wei Yuxia. Heterologous expression and immobilization of D-allulose 3-epimerase in Bacillus subtilis [D]. Wuxi: Jiangnan University, 2021.
[31] Hicks K B , Simpson G L , Bradburya G W.Removal of boric acid and related compounds from solutions of carbohydrates with a boron-selective resin (IRA-743) [J] .Carbohydr Res , 1986 , 147 (1) :39-48.
[32] Li Yunfei, Luan Qingmin, Liu Feng, et al. Research progress in the application of simulated moving bed chromatography in the separation of multicomponent sugar solutions [J]. Food and Fermentation Industry, 2022, 48(11): 1-13.
[33] Ma Pingying, Luo Wen, Zhan Yixin, et al. Research progress of Bacillus subtilis expression system [J]. Jiangxi Science, 2020, 38(6): 867-871.
[34] Ministry of Health of the People's Republic of China. GB 25584-2010 National Food Safety Standard Food Additives Magnesium Chloride [S]. Beijing: China Standards Press, 2010.
[35] Ministry of Health of the People's Republic of China. GB 29208-2012 National Food Safety Standard Food Additives Manganese Sulfate [S]. Beijing: China Standards Press, 2012.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






