What Is the Use of Astragalus Polysaccharide Extract?
Astragalus is the root of Astragalus membranaceus in the genus Astragalus, family Leguminosae. Astragalus polysaccharide is a substance proposed from Astragalus membranaceus, and the content of astragalus polysaccharide varies among different places of origin, with the highest content in Astragalus membranaceus produced in Gansu [1]. In recent years, astragalus polysaccharides have been found to have antioxidant[6], anti-apoptotic[7], anti-inflammatory[8], immunomodulatory[9], and antimicrobial[10] effects in pigs[2], chickens[3], and rats[4], as well as in vitro experiments[5] .

With the rapid development of pig farming and feed industry, “anti-reduction” has become a trend nowadays. As a green additive, astragalus polysaccharide can improve the production performance and avoid the environmental problems caused by antibiotics. Therefore, this paper summarizes the physiological functions and mechanisms of Astragali polysaccharides at home and abroad in recent years, and gives an overview of the application effects in swine production, with a view to providing references for the use of Astragali polysaccharides in pig breeding.
1 Physicochemical properties of Astragalus polysaccharide
Astragalus polysaccharide is the active ingredient in the traditional Chinese medicine Astragalus, yellow-brown in color, composed of rhamnose, arabinose, xylose, mannose, galactose and common sugar [11]. The molecular weight of Astragalus polysaccharide is between 80-160 kDa, which has obvious biological activity [12].
2 Physiological functions and mechanisms of action of Astragalus polysaccharides
2.1 Antioxidant properties
In the process of energy metabolism, the mitochondria in the cells will generate a large number of free radicals, which will cause various cellular damages. Astragali polysaccharides can directly or indirectly destroy free radicals and their oxidized products. 2.1.1 Direct Scavenging of Free Radicals
2.1.1 Direct scavenging of free radicals
In the presence of oxygen and metal ions (e.g., Fe2+ and Cu2+ ), mitochondria produce a group of strong oxidizing groups in energy metabolism, such as superoxide anion (O2- ), hydroxyl radical (OH- ), and 1,1-diphenyl-2-phenylhydrazine radical (DPPH- ). Ni Huiyan et al [13] found that astragalus polysaccharides had scavenging effects on DPPH-, OH-, and O2- in an in vitro antioxidant assay. The scavenging efficiency of astragalus polysaccharides in scavenging free radicals was concentration-dependent.
Luo Nan et al.[14] found that the concentration of Astragali polysaccharide solution increased from 0.1 mg/mL to 0.9 mg/mL, and the efficiency of OH- scavenging increased with the increase of the concentration of Astragali polysaccharide, and the highest scavenging efficiency was found at 0.9 mg/mL. However, it is not known whether the free radical scavenging ability of Astragali polysaccharide has a limit.
The scavenging of free radicals by astragalus polysaccharides is mainly caused by the following two aspects: OH- and O2- can combine with OH- and H+ provided by each monosaccharide group in astragalus polysaccharides to form stable compound water, thus scavenging free radicals [15- 16]; astragalus polysaccharides, as a kind of polysaccharide plant extracts, the OH- of polysaccharides can be complexed with Fe2+ and Cu2+, which are necessary for the generation of free radicals, thus reducing the generation of free radicals [17]. The OH- on polysaccharides can complex with Fe2+ and Cu2+, which are essential for the production of free radicals, thus reducing the production of free radicals [17]. However, the above studies were all conducted in vitro, and the in vivo scavenging of free radicals by Astragalus polysaccharides is still not perfect and needs further research.
2.1.2 Inhibit the level of reactive oxygen species (ROS) and increase the activity of peroxidase
Reactive oxygen species (ROS) are derivatives of free radicals. In the process of electron transfer in the mitochondrial respiratory chain, part of the oxygen is not fully oxidized to produce ROS [18]. It has been found that astragalus polysaccharide reduces the generation of ROS in myocytes[19] and endothelial cells[20] by maintaining the stability of the mitochondrial respiratory chain. When the content of ROS and free radicals increases in the body, the content of peroxides will also increase. Therefore, various peroxidases [e.g., superoxide dismutase (SOD), glutathione peroxidase (GSH- PX)] are produced in the body to scavenge excess peroxides and free radicals.
Xu Shengming et al [21] increased SOD and GSH-P1 activities in the blood of weaned piglets by 8.8% and 104.5%, respectively, with the addition of Astragalus polysaccharide to their diets. Chen Wei et al [22] found that astragalus polysaccharide could improve the dysfunction of peroxidase gene in transgenic SOD2+/- mice by increasing the expression level of SOD2mRNA and SOD2 activity. The mechanism is that astragalus polysaccharide activates adenylate-activated protein kinase (AMPK) to increase the expression level of peroxidase gene, and then increase the peroxidase activity [23]. However, the specific mechanism by which AMPK regulates peroxidase activity remains to be investigated.
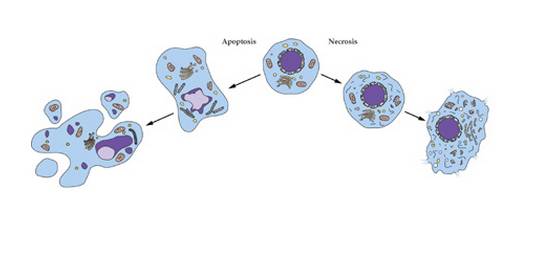
2.2 Anti-apoptosis
Apoptosis is mainly realized through the mitochondrial pathway, endoplasmic reticulum stress and death receptor pathway. In the present study, only the mitochondrial pathway and endoplasmic reticulum stress were found to have the anti-apoptotic function of Astragali polysaccharides.
2.2.1 Stabilization of mitochondrial membrane
The stability of mitochondrial membrane plays an important role in the process of apoptosis. Excessive Ca2+, B-cell lymphoma-2 protein (Bcl-2) and Bcl-2-related X protein (Bax) can alter the permeability of mitochondrial membranes, resulting in the release of cytochrome C from mitochondria into the cytosol, and ultimately activating the cysteine-aspartate protease caspase3 to induce apoptosis. Fan Zongjing et al.[24] found that astragalus polysaccharide could significantly reduce the concentration of Ca2+ in cardiomyocytes during transient ischemia and hypoxia, alleviate the damage of transient ischemia and hypoxia on the outer membrane of mitochondria, and avoid apoptosis of cardiomyocytes.
Sun et al.[25] found that astragali polysaccharide could inhibit apoptosis by enhancing the expression of Bcl-2 and inhibiting the expression of Bax, thus increasing the ratio of Bcl-2 to Bax in mitochondria, and thus inhibiting apoptosis.Liu et al.[26] found that astragali polysaccharides could have a certain protective effect on mitochondria in a Parkinson's disease mouse model, thus reducing the release of cytochrome C from mitochondria. Liu et al.[26] found that astragalus polysaccharide could have a certain protective effect on mitochondria, thus reducing the release of cytochrome C from mitochondria and avoiding apoptosis. Wei Pingting et al.[27] found that astragalus polysaccharide could significantly reduce the expression of caspase 3 protein in the renal tissue of rats affected by ephedrine, and reduce the apoptosis of renal tissue cells, thus slowing down the damage of ephedrine to the renal tissue of rats.
2.2.2 Endoplasmic Reticulum Stress (ERS)
Endoplasmic reticulum stress (ERS) is one of the major pathways of apoptosis, and ERS induces apoptosis mainly through C/EBP homol- ogous protein (CHOP), c-Jun amino- terminal kinase (JNK) pathway and caspase 12 [28]. apoptosis by c-Jun amino-terminal kinase (JNK) pathway and caspase 12 [28]. Under the strong effect of ERS, the increase of activated transcription factor (ATF)6 and the overexpression of PRKR-like endoplasmic reticulum kinase (PERK) caused the increase of CHOP, which can inhibit Bcl-2 to induce apoptosis. CHOP can inhibit Bcl-2 to induce apoptosis.
In a diabetic rat model, astragalus polysaccharide reduced CHOP protein synthesis by inhibiting ATF6 activation [29] and decreasing PERK expression [30] [31]. Prolonged ERS continuously activates inositol need enzymes (IER)1, which in turn transmits apoptotic signals to the JNK pathway to cause apoptosis [32]. Ouyang Jingping et al [33] found that astragalus polysaccharide could inhibit the activation of IER1 in a diabetic rat model. In a cardiac ischemia perfusion model, astragali polysaccharides increased the Bcl-2 to Bax ratio in cardiomyocytes by inhibiting the activation of JNK and prevented cardiomyocyte apoptosis [34]. Meanwhile, caspase 12, the initiator of apoptosis, can activate caspase 3 and induce apoptosis. In a diabetic rat model, astragali polysaccharide inhibited the expression of caspase 12 to reduce the apoptosis rate of cardiomyocytes[35] .
In summary, astragaloside can reduce apoptosis by maintaining the stability of mitochondrial membrane and reducing endoplasmic reticulum stress. However, the specific mechanism of astragalus polysaccharide in reducing apoptosis through the death receptor pathway needs to be further investigated.
2.3 Anti-inflammatory
Nuclear transcription factor-κ B (NF-κ B) plays an important role in inflammation. Under the stimulation of inflammatory mediators, inhibitory protein-κ B (Iκ B) in the cytosol is phosphorylated and detached from NF-κ B, thus activating NF-κ B [36]. Activated NF-κ B can enter the cell nucleus and regulate the expression of a series of pro-inflammatory genes, thus enhancing the inflammatory response [37]. It has been found that astragalus polysaccharide can inhibit the activation of NF-κ B in cells [38] and prevent the phosphorylation of Iκ B [39], reduce the level of activated NF-κ B in cells, and thus slow down inflammation. Cheng Yan[40] and others found that astragalus polysaccharides inhibited the expression of inflammatory mediators and reduced the release of inflammatory mediators in a mouse model of sepsis, thus preventing the damage of sepsis to cardiomyocytes. Some proteases are also involved in the inflammatory process, such as cyclooxygenase (COX), which catalyzes the production of prostaglandins from arachidonic acid, allowing inflammation to spread. Liu Ling et al.[41] found that astragalus polysaccharides inhibit the expression of the COX gene, thus reducing the production of COX enzyme and playing an anti-inflammatory role.
In summary, astragalus polysaccharide can be used as an anti-inflammatory agent in three ways: first, it can reduce the level of activated NF-κ B, thus reducing the production of pro-inflammatory mediators; second, it can inhibit the expression of inflammatory mediators, thus lowering the level of inflammatory mediators; and third, it can change the activity of inflammatory enzymes through the regulation of the genes of inflammation-related enzymes, thus exerting its effect of inhibiting inflammation.
2.4 Regulation of immunity
2.4.1 Enhancing the development of immune organs
The strength of an animal's immunity is determined by the development of its immune organs (e.g. spleen, thymus, bursa). Xiang Shuangyun et al. [3] significantly increased the spleen and bursa indices of laying hens by injecting them with Astragalus polysaccharide for 3 consecutive days before vaccination against Newcastle disease. Zhang et al. [42] found that feeding Astragali polysaccharide solution to rats vaccinated against foot-and-mouth disease virus stimulated the proliferation of rat spleen cells, and LI et al. [43] found that injecting Astragali polysaccharide solution into pigs vaccinated against foot-and-mouth disease virus significantly increased the quality of their spleens.
2.4.2 Enhancement of immune cells
Immune cells mainly include T cells, B cells, macrophages and so on. Among them, T cells are the main cells in cellular immunity, which are mainly divided into two subpopulations-CD4+ and CD8+ according to their functions [44], and the ratio of CD4+/CD8+ can be used to measure the immunity performance of the body [45]. It was found that astragalus polysaccharide increased the number of T cells by increasing the CD4+/CD8+ ratio in breeding sows, which in turn improved the immune performance of breeding sows [9]. Wang Chaofeng et al.[46] showed that the addition of astragalus polysaccharide to diets significantly increased the CD4+ in the blood of weaned piglets, which in turn increased the CD4+/CD8+ ratio and enhanced the immunity of piglets.
Hou Xi'e et al.[47] injected lactating piglets with astragali polysaccharide solution and found that astragali polysaccharide reduced CD8+ in piglets and induced B cells to secrete more globulins. γ-Interferon-γ (IFN-γ ) is a water-soluble glycoprotein, produced by activated T cells. IFN-γ can promote T cell differentiation and enhance cellular immunity by up-regulating the transcription factor T-bet. Yao Jingming et al [48] injected pigs vaccinated against swine fever with astragalus polysaccharide solution, which could significantly increase the IFN-γ content in pigs vaccinated against swine fever, promote T cell differentiation, and then enhance cellular immunity. Toll-like receptors (TLR4) play an important role in the immune function of phagocytes, and it has been found that the polysaccharide structure of astragalus polysaccharides activates phagocytes by binding to TLR4 on the surface of phagocytes, thus stimulating the secretion of immune-related effectors by phagocytes, and thus enhancing the immune ability of phagocytes [49-51].
2.5 Antibacterial
Astragalus polysaccharide has antibacterial properties [10]. In vitro antimicrobial tests showed that astragalus polysaccharides inhibited Escherichia coli, Streptococcus and Staphylococcus aureus, but the inhibitory effect was most obvious in E. coli [52]. Xie Hongbing et al [53] added 800 mg/kg astragalus polysaccharide to the diet of weaned piglets and found that the number of E. coli in the intestinal tract of weaned piglets in the astragalus polysaccharide group was significantly reduced compared with that of the control group.Li [54] et al. added 200 mg/kg astragalus polysaccharide to the diet of 1-day-old hens and carried out a feeding trial for 42 days, and found that the number of E. coli in the ileum and the cecum of the hens was reduced by 4% and 6%, respectively.5
The antibacterial effect of astragalus polysaccharide on the intestinal tract of 1-day-old hens was also found to be significant. The main mechanism of antibacterial effect of astragalus polysaccharide is as follows: astragalus polysaccharide promotes the development of intestinal villi, increases the number of tonsils and lymph nodes in the intestine, and enhances intestinal immune function, which in turn induces the proliferation of Lactobacillus and Bifidobacterium in the intestinal tract [55]. As a result, the production efficiency of Lactobacillus and Bifidobacterium in the decomposition of food to produce organic acids is greatly increased, which in turn lowers the pH in the intestine to inhibit the growth of pathogenic bacteria in the intestine [56]. In summary, in the animal in vivo test, the effect of Astragali polysaccharide on E. coli was significant, but there is a lack of accurate data on the inhibitory effect on other pathogenic bacteria, which needs to be investigated.
3 Effects of Astragalus polysaccharide on pig production
3.1 Effect on growing pig production
From Table 1, it can be seen that adding Astragalus polysaccharide to feed can improve the production performance of growing pigs. The mechanism is mainly caused by two aspects, on the one hand, astragalus polysaccharide can promote the growth of intestinal epithelial cells and small intestinal villi, thus promoting the development of the intestinal tract, and directly improve the efficiency of intestinal digestion and absorption of nutrients [60]; on the other hand, astragalus polysaccharide to increase the number of beneficial bacteria, so that the beneficial bacteria decomposition of carbohydrates into the intestinal tract can be easily absorbed by the organic acid, thereby indirectly promoting the intestinal tract to the absorption of nutrients [56].
On the other hand, astragalus polysaccharide increases the number of beneficial bacteria, which makes them decompose carbohydrates into organic acids, thus indirectly promoting the intestinal absorption of nutrients [56]. However, when the amount of astragalus polysaccharide is added to the diet is too large, astragalus polysaccharide will increase the viscosity of the chow in the gastrointestinal tract, reduce the interaction between digestive enzymes and chow, and then reduce the digestibility, which will ultimately affect the production performance of growing pigs [61]. To summarize, adding suitable concentration of astragalus polysaccharide to the diet can improve the performance of growing pigs.

3.3 Effects on boar semen
It was found that the addition of 0.3 mg/mL astragalus polysaccharide to the cryodilution solution could significantly improve the preservation effect of boar semen cryopreservation [67]. Liu Ying et al. [68] added 0.04 mg/mL, 0.06 mg/mL, 0.08 mg/mL, 0.10 mg/mL of Astragalus polysaccharides to the cryodilution solution and found that 0.08 mg/mL of Astragalus polysaccharides had the best preservation effect on boar semen. Because the freezing process can induce the production of ROS in porcine spermatozoa and lipid peroxidation of unsaturated fatty acids in the plasma membrane, resulting in oxidative damage [69], astragalus polysaccharide can increase the activity of catalase and its mRNA expression in spermatozoa [70], and at the same time, it can eliminate the excessive ROS in the spermatozoa mitochondria [71], which can increase the spermatozoa's ability to resist oxidative stress. However, different scholars have different opinions on the appropriate concentration of astragalus polysaccharide. This may be due to the different molecular weights of astragalus polysaccharides. The origin, extraction method and molecular weight of astragalus polysaccharides should be considered when adding astragalus polysaccharides to feeds. Detailed data on the effect of astragalosides on semen formation are not available.
4 Summary
In summary, astragalus polysaccharides have specific physiological functions, and their application in swine production has achieved preliminary results in recent years. In order to better meet the future goal of “antimicrobial-free farming” and to better utilize astragalosides, in-depth studies are needed in the following aspects: 1) to explore a new extraction method of astragalosides and to improve the efficiency of astragaloside extraction; 2) to investigate the appropriate concentration of astragalosides in feed for different breeds of pigs and at different stages of growth and to study the side effects and their mechanisms caused by excessive additions; 3) to study the effect of astragalosides on the diets of pigs of different breeds and growth stages. 2) Explore the appropriate concentration of astragalus polysaccharide in feed for different breeds of pigs and different growth stages, and study the side effects caused by over-addition of astragalus polysaccharide and its mechanism; 3) Explore the effect and mechanism of the combined application of astragalus polysaccharide with other plant polysaccharides, probiotic bacteria, and traditional Chinese medicines to improve the performance of pigs.
References:
[1] ZHU Shangwen, WEI Jia, XU Yongzhen, et al. Contents of astragalus polysaccharides in Astragalus from different origins and their anti-fatigue and normobaric hypoxia-resistant effects[J]. Journal of Shaanxi University of Traditional Chinese Medicine, 2016,39(1):86-89.
[2] LI Xingru,LI Shaowei,ZHANG Yamei,et al. High-dose swine fever vaccine combined with astragalus polysaccharide for the treatment of hyperthermia in pigs[J]. Journal of Chinese Veterinary Medicine,2011,13(6):63-63.
[3] XIANG Shuangyun, ZHOU Zhenhui, CAO Jinyuan. Effects of astragalus polysaccharide on the immunization effect of chicken Newcastle disease vaccine[J]. Feed Research,2017(24):38-41.
[4] HAN Lin,WANG Hongxin,RU Meiwei. Astragalus polysaccharide attenuates LPS-induced apoptosis in mouse cardiomyocytes by inhibiting NF-κB and JNK signaling pathways[J]. Chinese Pharmacology Bulletin,2018,34(2):243-249.
[5] MA Jinyun,WANG Jinying,SUN Yu,et al. Experimental study on inhibition of EAE and modulation of BV-2 neuromicroglia activation by astragalus polysaccharide in mice[J]. Chinese Journal of Immunology, 2018,34(3):381- 387.
[6] Yang F,Yan G,Li Y,et al. Astragalus Polysaccharide Attenuated Iron Overload -Induced Dysfunction of Mesenchymal Stem Cells via Suppressing Mitochondrial ROS.[J].Cellular Physiology & Biochemistry, 2016,39(4):1369-1379.
[7] Zheng J, Ma L T, Ren Q Y,et al.The influence of astragalus polysaccharide and β-elemene on LX-2 cell growth, apoptosis and ac- tivation[J].Bmc Gastroenterology,2014,14(1):224.
[8] Yue S, Jian L, Lei W,et al. Improving Effects of Astragalus Polysac - charides on Cardiac Function via Keap1/Nrf2-ARE Signal Pathway in Adjuvant Arthritis Rats [J].Chinese Herbal Medicines,2016,8 (2):143- 153.
[9] LIU Xinping, CHEN Chong, FU Shuguang, CHEN Ling, YUAN Jiuxiang. Improvement of immunosuppressive effect of astragalus polysaccharide in swine fever mixed-susceptible sows[J]. Journal of Jiangxi Normal University of Science and Technology,2015(6):36-39.
[10] SUN Bo, CHEN Jing, LIU Jiang, et al. Effects of adding astragalus polysaccharide to feed on intestinal flora and immunity organ index of broilers[J]. Heilongjiang Animal Husbandry and Veterinary Medicine,2014(13):86-88.
[11] LIU Yanzhong , ZHANG Fujun . Introduction to the application of veterinary drug astragalus polysaccharide[J]. Modern Animal Husbandry Science and Technology , 2010(6):210-210.
[12] JIANG Guangzhi . Research on the immunomodulation and resistance to blue ear virus infection by Astragalus polysaccharides[D]. Hefei:Anhui Agricultural University,2015.
[13] Ni Huiyan, Chen Wei, Song Wenjing . Study on the antioxidant effect of Astragalus polysaccharide[J]. Journal of Chinese Medicine , 2017,32(9):1705-1707.
[14] Luo N, Xu LY, Ruan HH, et al. Study on the antioxidant activity of Astragalus polysaccharide[J]. Research on the antioxidant activity of astragalus polysaccharides[J].
[15] SUN Chengwen, JIANG Yan, ZHONG Guo Gan, et al. Study on the antioxidant damage of Astragalus polysaccharide[J]. Chinese Journal of Pharmacology,1996(2):161-163.
[16] WANG Jianhang, LI Yu, LIU Anjun. Extraction and antioxidant activity of polysaccharides from Pinus sylvestris [J]. Food Research and Development,2006,27(11):53-56.
[17] ZHAO Linjing, SONG Xiaoping, LAI Fangya. Progress of research on antioxidant properties of polysaccharides and their derivatives[J]. Journal of Shanghai University of Engineering and Technology,2008(1):44-47.
[18] GENG Junwei, YU Han, LIN Zhi. Generation and metabolism of reactive oxygen species in animal cells[J]. Life Science,2015,27(5):609-617.
[19] Huang Y F,Lu L,Zhu D J,et al.Effects of Polysaccharides on Dys - function of Mitochondrial Dynamics Induced by Oxidative Stress [J]. Oxidative Medicine & Cellular Longevity,2016(10):1-13.
[20] Han, Tang, Lu, et al. Astragalus polysaccharide ameliorates H2O2 -induced human umbilical vein endothelial cell injury [J]. Molecular Medicine Reports, 2017, 15(6):4027-4034.
-
Prev
What Are the Uses of Astragalus Extract Astragalus Polysaccharides?
-
Next
What Are the Uses of Blueberry Fruit in Tamil?


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese




