What Is the Prepare Method of D Tagatose?
D Tagatose is a rare six-carbon ketose sugar in nature. Its physical properties and sweetness are similar to sucrose. It has low energy content, can lower blood sugar, improve intestinal flora, and prevent tooth decay. Foreign researchers have studied its physiological functions and production methods in more detail, and D-tagatose has been used as a low-calorie sweetener in health drinks, yogurt, fruit juice, and foods for diabetics in many countries. In 2001, the US Food and Drug Administration (FDA) determined that it was generally recognized as safe (GRAS) [1]. There has been little research on tagatose in China, and there are many problems with its industrial production and its application also needs to be studied. However, the number of people with diabetes and cardiovascular diseases is increasing year by year in China, and the demand for functional sweeteners is also growing. Therefore, D-tagatose has great market potential in China.
1 D-tagatose
1. 1 Physical and chemical properties
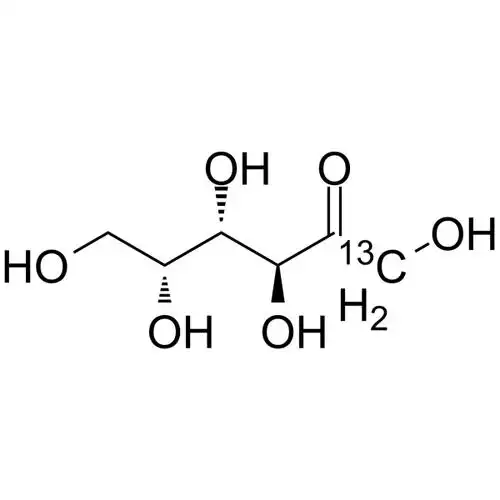
D-tagatose is an isomer of D-galactose and a diastereoisomer of D-fructose (see Figure 1), with a relative molecular mass of 180.16 u. Pure D-tagatose is a white, odorless, non-crystalline substance with a melting point of 134 °C. It is stable in the pH range of 2 to 7. It is highly soluble in water, with a solubility of 58% at 21°C. Its hygroscopicity is similar to that of sorbitol, and its viscosity of 180 cP (70% (w/w), 20°C) is lower than that of sucrose, slightly higher than that of sorbitol and fructose [2]. The sweetness is similar to that of sucrose, with a sweetness level of 92% of sucrose, and the calories produced are only 1/3 of sucrose. The U.S. FDA has confirmed it as a low-calorie sweetener with an energy value of 1.5 kcal/g (approximately 6280.2 J/g). In addition, D-tagatose is prone to the Maillard reaction and can caramelize at lower temperatures.
1. 2 Physiological function
(1) With low energy content, D-tagatose can be catabolized via the tagatose-6-phosphate pathway, which is present in some microorganisms but not in higher animals [3]. The absorption rate of D-tagatose in the small intestine is very low. The part that is not absorbed by the small intestine reaches the large intestine and is completely fermented by the intestinal microorganisms, producing a large amount of short-chain fatty acids that are almost completely absorbed and metabolized. The fermentation process produces relatively low amounts of energy, and there is also a loss of energy due to the increased excretion of microbial waste products. Therefore, the energy produced by the catabolism of tagatose is much lower than that of sucrose. If tagatose is used to replace sucrose in the diet, it can effectively reduce the incidence of obesity.
(2) Lowering blood sugar: Studies have shown that there is no significant change in blood sugar or insulin levels after ingesting tagatose. Tagatose also inhibits the absorption of glucose in the small intestine, which can effectively reduce the blood sugar rise caused by glucose intake in diabetic patients, and has a role in adjuvant therapy for type 2 diabetic patients.
(3) Improves intestinal flora. D-tagatose is selectively fermented in the colon by some microbial flora, promoting the growth of beneficial bacteria. It is a good prebiotic. At the same time, the large amount of beneficial short-chain fatty acids produced by the fermentation of D-tagatose has a good effect in inhibiting colon cancer, inhibiting intestinal pathogenic bacteria, and promoting the growth of beneficial bacteria [3]. Therefore, D-tagatose can improve intestinal flora and maintain intestinal health.
(4) Anti-caries: D-tagatose is similar to polyols in protecting teeth. Because it produces low levels of acid in the mouth and does not lower the pH of plaque, it can effectively prevent the occurrence of caries and enamel erosion [4].
2 D-Tagatose biosynthesis
D-Tagatose can be produced by bioconversion or chemical synthesis. Because chemical production of D-Tagatose is prone to the formation of impurities such as fructose, sorbitol and mannose, which require repeated crystallization to remove, this significantly reduces the yield of D-Tagatose. Therefore, bioconversion is being continuously researched as a better method.
The bioconversion method for producing tagatose mainly uses L-arabinose isomerase to catalyze the conversion of D-galactose to D-tagatose. The natural function of L-arabinose isomerase (EC 5.3.1.4, L-arabinose isomerase, L-AI) is to catalyze the conversion between aldose and ketose mutual conversion [5]. Research has found that it can also catalyze the conversion of D-galactose to D-tagatose, but its affinity for D-galactose is lower than its affinity for L-arabinose.
2.1 L-AI sources
The optimal reaction conditions for L-AI vary depending on the source. The optimum reaction temperature for L-AI from mesophilic bacteria is 30 to 50 °C, including Alicyclobacillus acidocaldarius, Bacillus halodurans, Escherichia coli, and Lactobacillus gayon-ii. The optimum reaction temperature for L-AI from thermophilic bacteria is 60 to 80 °C, including Geobacillus stearothermophilus, G. thermodenitrificans, and Thermoanaerobacter mathrani. The optimum reaction temperature for L-AI from hyperthermophilic bacteria is 85-90°C, including Thermo- toga neapolitana and T. Maritima. As the conversion rate of L-AI to D-tagatose increases with increasing temperature, most of the previous L-AI sources were thermophilic bacteria, such as Bacillus stearothermophilus US100 and Thermoanaerobacter mathrani. However, these thermophilic bacteria are not food-grade microorganisms, and their food safety is questionable. Therefore, researchers have begun to use food-grade microorganisms to produce D-tagatose.
Humans have been using lactic acid bacteria to produce fermented foods such as various dairy products for thousands of years. Lactococcus lactis is currently one of the best Generally Recognized as Safe (GRAS) organisms, and it is also an effective expression host for many different protein products. Moreover, the pH at which lactic acid bacteria grow is the same as that at which lactose is hydrolyzed, making them an excellent choice for the bioconversion of D-tagatose. Currently, food-grade strains of lactic acid bacteria that have been shown to express L-tagatose include Lactobacillus gayon-ii [6], Lactobacillus plantarum [7], Lactobacillus sakei 23K [8], and Lactobacillus fermentum, which was discovered in 2011 [9]. (Lactobacillus sakei 23K) [8] and Lactobacillus fermentum [9], a food-grade strain discovered in 2011. The use of food-grade strains makes the microbial enzymatic production of tagatose safer.
2. 2 Molecular modification of L-AI
Although the araA gene (which expresses the L-AI protein) has been identified in many species, there are still many problems with the industrial production of L-AI. Therefore, molecular modification of L-AI to obtain an enzyme that meets the requirements of industrial production has become an important part of L-AI research. The crystal structure of E. coli L-AI has been determined, providing a basis for identifying the molecule responsible for the isomerization of galactose to tagatose. Rhimi et al. [10] determined the essential catalytic and substrate-recognition sites of L-arabinose isomerase from G. stearoothermophilus US100 based on the crystal structure and sequence. In order to improve the conversion rate of D-galactose, the molecular modification of L-AI mainly focuses on improving substrate specificity, heat resistance, and reducing the optimal pH [11].
Direct evolution of the L-AI gene was considered the most effective method to improve the reaction rate [12]. The researchers obtained a mutant L-AI from G. stearothermophilus through polymerase chain reaction. The enzyme had three amino acid site changes compared to the wild enzyme, V322M, A393T and A408V. This variant of L-AI has improved catalytic activity towards D-galactose, optimum temperature, catalytic efficiency and yield of D-tagatose [13]. The research group of Deok-Kun Oh carried out site-directed mutagenesis on L-AI from G. thermodenitrificans to obtain an enzyme with two mutant sites (C450S-N475K). AI from G. thermodenitrificans was subjected to site-directed mutagenesis to obtain a double mutant enzyme (C450S-N475K). This double mutant enzyme had a yield of D-tagatose of 58%, compared to 46% for the wild-type enzyme [14].
Studies have shown that Mn2 + and/or Co2 + are necessary for the activity and thermal stability of many L-AI. However, in the production of D-tagatose by the biological enzyme method, the addition of high concentrations of metal ions will also increase the cost of post-processing. Therefore, the search for metal ion-independent L-AI with thermal stability has also become a major direction of L-AI molecular modification. At present, the three-dimensional structure of E. coli L-AI has been determined, and a possible metal binding site has been speculated by comparing its crystal structure with the crystal structure of E. coli L-trehalose isomerase [15].
The industrial production of D-tagatose requires L-AI to react in the acidic pH range. Because D-tagatose is stable at pH 2 to 7, acidic conditions can reduce browning reactions. Moreover, lactose is usually used as a raw material in production, and lactose needs to be hydrolyzed to galactose first, and lactose hydrolysis usually occurs under acidic conditions (pH 5 to 6). Therefore, the use of acidic L-AI conversion to produce D-tagatose can eliminate the need for pH adjustment and reduce costs. The acquired acid-tolerant L-AI includes two mutants, Q408V and R408V (pHopt 7.5), obtained from a GSAI (pHopt 8.5) site-directed mutation [16]. At present, the amino acid sites that affect pHopt can be determined, including Val408 (GSAI) and Lys269 (AAAI, corresponding to Glu268 of BHAI and Gln268 of BSAI). In the future, these two sites can be mutated or other amino acid sites affecting pHopt can be found based on the crystal structure of L-AI.
2. 3 Expression of L-AI
At present, Escherichia coli is often used as a host cell to produce L-AI. However, the production of endotoxins in E. coli may pose a safety problem. Therefore, after obtaining the L-AI gene suitable for industrial production and application, expressing it in food-grade genetically engineered bacteria has become a new research focus. Xu et al. [17] used the Lactobacillus fermentum CGMCC2921 instead of Escherichia coli as the expression vector for L-AI and achieved the large-scale expression of L-AI. Noora et al. [18] transferred the L-AI gene to the lactic acid bacteria Lactococcus lactis, enabling the expression of L-AI in a phosphate depletion-induced expression system.
2. 4 Production of D - Tagatose using immobilized biocatalysts
After obtaining engineered bacteria with high L - AI expression, immobilization of the enzyme or the enzyme-producing cells is required to improve the mechanical strength of the enzyme and reduce the loss of enzyme activity. The Oh research group at Seoul National University in South Korea used different immobilization methods such as silica gel adsorption, microencapsulation, sodium alginate embedding, and glutaraldehyde cross-linking to immobilize Escherichia coli L-AI and compare the effects of different immobilization methods on the conversion rate of D-tagatose. The results showed that the enzyme preparation obtained by using the sodium alginate-calcium chloride method to obtain immobilized beads and then cross-linked with glutaraldehyde had the best effect.
By comparing the ability of free enzymes, immobilized enzymes and immobilized cells to produce D-tagatose, the researchers found that using the same amount of cells, the yield of D-tagatose produced by immobilized cells was the highest. Moreover, using L-AI producing cells as catalysts can better protect the enzyme, prevent denaturation of the enzyme, increase the number of reaction batches, and reduce impurities in the conversion solution. Fu Fenggen et al. [19] studied the ability of immobilized recombinant E. coli cells to produce D-tagatose. The results showed that using D-tagatose as a substrate under optimal reaction conditions for 24 hours, the immobilized the highest conversion rate of D-tagatose, up to 65.8%, and the average conversion rate of 60.6% for 8 consecutive batches, laying a foundation for the industrial production of D-tagatose.
2.5 Effect of boric acid on the yield of D-tagatose
In addition to increasing the reaction temperature, another way to shift the reaction equilibrium towards D-tagatose is to add boric acid [B(OH)4-] to the reaction mixture. Boric acid forms complex complexes with different sugars, generally showing a greater affinity for ketoses than for aldoses. This property has been exploited, for example, to enhance the ketose formation from aldoses in the chromatographic separation of carbohydrates. Borate salts have been shown to form a tighter complex with ketoses, for example binding more readily with D-tagatose than with D-galactose, with L-ribulose than with L-arabinose, and with D-allulose than with D-fructose. In addition to increasing the conversion, the presence of a borate buffer can also increase the reaction rate.
It has been reported that D-tagatose, L-ribulose and -D-allulose have maximum conversion rates of 74, 89 and 64%, respectively, in the presence of boric acid. Adding boric acid to the ketose production process can break the original chemical equilibrium and increase the yield of the target product. The boric acid in the conversion solution is removed from a carbohydrate-borate complex using a special borate ion exchange column [20], which does not affect the purity of the product. According to Noora et al. [18], the yield of D-tagatose was 74% using L-AI derived from Thermotoga neapolitana at 60°C, pH 9.0, and with the addition of boric acid, which was 24% higher than the control group without boric acid. Fu Fenggen et al. [19] investigated the effect of the molar ratio of boric acid to substrate on the yield in the isomerization reaction system in a study on the production of D-tagatose by immobilized recombinant Escherichia coli cells. The results showed that the addition of an appropriate amount of boric acid can change the original chemical equilibrium and achieve high yields of D-tagatose.
2. 6 Separation and purification of D-tagatose
Both the bioconversion and chemical conversion methods use D-galactose as the raw material, and the final reaction product is a mixture of D-tagatose and D-galactose. Therefore, the separation and purification of D-tagatose is also a factor affecting the yield of D-tagatose.
The commonly used separation methods include cation exchange chromatography or simple resin separation. Huang Wenxia et al. [21] used Ca2 + ion exchange resin chromatography and anion and cation exchange resin desalination and decolorization to separate and purify D-tagatose. The recovery rate of D-tagatose reached 83%, and the purity reached 98. The principle of separation is mainly based on the difference in the degree of complexation of different monosaccharides with Ca2 + to separate and purify the monosaccharides. It has also been reported in the literature that D-tagatose can be purified by selectively degrading D-galactose using a beer yeast cell (Saccharomyces cerevisiae L1). The purity of D-tagatose obtained by this method can reach more than 95%. Although unreacted D-galactose cannot be recovered for reuse, this method has the advantages of high separation efficiency, low cost and simple operation, providing more options for the industrial production of D-tagatose.
3. Applications of D-tagatose
3.1. Applications in food
Because D-tagatose has physical properties and sweetness similar to sucrose, and also has physical and chemical properties such as acid resistance, alkali resistance and heat resistance, it has broad application prospects in the food industry as a functional sweetener. It can be used in healthy drinks, yogurt, chocolate, chewing gum, foods for diabetics, diet foods, cereal foods, etc.
Currently, the main sweeteners commonly used in the beverage industry are cyclamate, aspartame, saccharin, acesulfame, stevia, etc. These are all strong sweeteners that are prone to producing undesirable aftertastes such as metallic, bitter and astringent flavours. However, the addition of tagatose does not cause any undesirable aftertastes. In addition, D-tagatose is a good prebiotic that can be fermented and utilized by probiotics, promoting the growth of probiotics such as Lactobacillus casei and Lactobacillus rhamnosus.

Studies have shown that D-tagatose can promote the growth of Lactobacillus casei and Lactobacillus rhamnosus, improve their beneficial activity and survival rate in the intestine. Therefore, D-tagatose can be used in probiotic supplements and also in yogurt, where it provides sweetness while increasing the number of live bacteria in the yogurt, enhancing its nutritional value and giving it a richer, fuller flavor. In 2001, the US Food and Drug Administration officially approved the use of D-tagatose as a sweetener in the food and beverage industry. In 2003, PepsiCo began using tagatose in Sprite drinks, and since then it has been widely used in the United States as a substitute for sucrose in healthy drinks, yogurt, fruit juice and other products.
D tagatose has the characteristic of being prone to caramelization at low temperatures. Studies have found that D-tagatose undergoes a Maillard reaction with amino acids to produce volatile substances such as 2-acetylfuran, 2-ethylpyrazine, 2-acetylthiazole and other volatile substances than reducing sugars such as glucose and galactose[22]. It is used in baked goods to not only produce an ideal color, but also a more mellow flavor. Because tagatose has lower viscosity than sucrose and is easily crystallized, if it is used to make icing and applied to the surface of cereal foods, it can increase the sweetness of the product and extend its shelf life.
3. 2 Applications in medicine, cosmetics and other fields
D-tagatose can be used in medicine as a treatment for type 2 diabetes. Studies have shown that D-tagatose can reduce the symptoms of type 2 diabetes by reducing body weight and increasing the content of high-density lipoprotein (HLP). It can also be used in cough syrup, denture adhesives and oral disinfectants. D-tagatose is used in cosmetics as a stabilizer and moisturizer. Because D-tagatose is effective against tooth decay and bad breath, it can be used in toothpaste and mouthwash. Currently, many toothpastes use D-sorbitol or glycerin, or both, as humectants. However, D-sorbitol is only half as sweet as sucrose, while D-tagatose is as sweet as sucrose and has similar hygroscopicity to sorbitol. Adding D-tagatose to toothpaste and mouthwash can enhance sweetness and meet taste requirements while maintaining good wettability and stability.
Reference
[1] Levin G V . Tagatose , a new GRAS sweetener and health product [ J] . J Med Food,2002,5 :23 - 36 .
[2 ] Oh D K . Tagatose : propertiea , application , and biotechnological processes [ J] . Appl Microbiol Biotechnol,2007 ,76:1 - 8 .
[3 ] Mu Wanmeng, Zhang Tao, Jiang Bo, et al. D - Research progress on tagatose and L-arabinose isomerase [J] . Food and Fermentation Industry, 2007, 33: 84-90.
[4 ] Wong D . Sweetener determined safe in drugs , mouthwashes , and toothpastes [ J] . Dent Today,2000,19:32,34 - 35 .
[5 ] Liang Min, Zhai Yafei, Zou Yang, et al. Application and production of the new sweetener tagatose [J]. Food and Drugs, 2011, 13: 125-128.
[6] Cheetham P S , Wootton A N . Bioconversion of D - galactose into D- tagatose [ J] . Enzyme Microb Technol,1993,15:105 - 108 .
[7 ] Chouaye k h H , Bejar W , Rhimi M , et al . Characterization of an L arabinose isomerase from the Lactobacillus plantarum NC 8 strain showing pronounced stability at acidic pH [ J ] . FEMS Microbiol Lett,2007 ,277 :260 - 267 .
[8 ] Rhimi M , Ilhammami R , Bajic G , et al . The acid tolerant L arabinose isomerase from the food grade Lactobacillus sakei 23 K is an attractive D-tagatose producer [ J ] . Bioresour Technol , 2010, 101:9171 - 9177 .
[9] Xu Z , Qing Y J , Li S , et al . A novel L-arabinose isomerase from Lactobacillus fermentum CGMCC2921 for D-tagatose production : gene cloning , purification and characterization [ J] . J Mol Catal B : Enzym,2011,70:1 - 7 .
[10] Rhimi M , Juy M , Aghajari N , et al . Probing the essential catalytic residues and the substrate affinity in the thermoactive Bacillus stearothermophilus US100 L-arabinose isomerase by site-directed mutagenesis[ J] . J Bacteriol 2007 ,189:3556 - 3563 .
[11] Kim P . Current studies on biological tagatose production using L -arabinose isomerase : a review and future perspective [ J ] . Appl Microbiol Biotechnol,2004 ,65 :243 - 249 .
[12 ] Kim P , Yoon S H , Seo M J , et al . Improvement of tagatose conversion rate by genetic evolution of thermostable galactose isomerase [ J] . Biotechnol Appl Biochem 2001,34 :99 - 102 .
[13 ] Kim H J , Kim J H , Oh H J , et al . Characterization of a mutated Geobacillus stearothermophilus L-arabinose isomerase that increases the production rate of D-tagatose [ J] . J Appl Microbiol , 2006,101:213 - 221 .
[14 ] Oh H J , Kim H J , Oh D K . Increase in D-tagatose production rate by site-directed mutagenesis of L-arabinose isomerase from Geobacillus thermodenitrificans [ J ] . Biotechnol Lett 2006 , 28:145 - 149 .
[15 ]Oh D K . Tagatose : properties , applications , and biotechnological processes [ J] . Appl Microbiotechnol,2007 ,76:1 - 8 .
[16 ] Oh D K , Oh H J , Kim H J , et al . Modification of optimal pH in L arabinose isomerase from Geobacillus stearothermophilus for D - galactose isomerization [ J] . J Mol Catal B-Enzym,2006,43 :108 - 112.
[17 ] Xu Z , Li S , Fu F , et al . Production of D-tagatose , a Functional Sweetener,Utilizing Alginate ImmobilizedLactobacillus fermentumCGMCC2921Cells [ J ] . Appl Biochem Biotechnol , 2012,166 :961 - 973 .
[18 ] Noora S , Kalle S , Matti L , et al . D-Tagatose production in the
presence of borate by resting Lactococcus lactis cells harboring Bifidobacterium longum L-arabinose isomerase [ J ] . Bioprocess Biosyst Eng,2013 ,36:489 - 497 .
[19 ] Fu Fenggen, Xu Zheng, Li Guixiang, et al. Production of D-tagatose using immobilized recombinant Escherichia coli cells [J]. Chinese Journal of Bioengineering, 2011, 31: 85-90.
[20 ] Hicks K B , Simpson G L , Bradbury A G . Removal of boric acid and related compounds from solutions of carbohy -drates with boron - selective resin ( IRA - 743 ) [ J ] . Carbohydr Res ,1986, 147 :39 - 48 .
[21] Huang Wenxia, Mu Wanmeng, Jiang Bo. Separation and purification of D-tagatose [J]. Food and Fermentation Industry, 2008, 34: 168-171.
[22] In H D , Sarah L , Hae-Roung J , et al . Comparison of Volatile Maillard Reaction Products from Tagatose and Other Reducing Sugars with Amino Acids [ J ] . Food Sci . Biotechnol ,2010, 19: 431 - 438 .


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese




