What Is the Character and Use of Natural Coloring?
Natural coloring is the general term for colored substances found in nature, mainly from the flowers, leaves, fruits and seeds of plants, as well as a small proportion from insects and microorganisms. Natural coloring is often used to improve the color of food, and is also widely used in medicine, cosmetics and fabric coloring. Natural Coloring has the following characteristics: most of it is obtained from plants and animals that people eat every day, it is safe, non-toxic and has no side effects; it is easily absorbed by the human body and is a beneficial supplement for maintaining human health; most Natural Coloring has physiological and pharmacological effects and can prevent and treat certain diseases [1]; it has a soft color and an aromatic smell that brings a feeling of pleasure. Natural Coloring has therefore always been popular, especially nowadays when there are frequent safety incidents involving chemically synthesized pigments, and people are eager to truly enjoy natural pigments in their daily lives. The author reviews the progress in research on the physical and chemical properties, isolation and purification, and applications of Natural Coloring.
1 Types and physical and chemical properties of Natural Coloring
In addition to mineral pigments, Natural Coloring can be divided according to its source into animal pigments, plant pigments and microbial pigments; according to its solubility, it can be divided into water-soluble pigments and fat-soluble pigments; it can also be divided according to its chemical structure or color series [2]. The following mainly introduces the classification according to chemical structure: (1) isoprene pigments (fat-soluble pigments): pigments with isoprene as the basic unit composed of a long chain of conjugated double bonds, such as lycopene, capsanthin and zeaxanthin; (2) pigments derived from pyrrole: based on porphyrin (consisting of 4 pyrrole rings), such as chlorophyll; (3) phenol, ketone and quinone pigments (alcohol-soluble and water-soluble): anthocyanins and tannins, such as geranium pigment and petunia pigment; (4) indole pigments: jujube pigment and red date pigment. The following mainly introduces several natural colorings that are closely related to people's production, life and health, as well as their physical and chemical properties.
1.1 Lycopene
Lycopene is mainly found in tomatoes (the content varies with the variety and maturity), and is also found in high concentrations in other fruits and vegetables such as watermelons, red peppers and grapefruit. It is a bright red needle-like crystal that belongs to the isoprene class of natural coloring. It is a fat-soluble pigment that is easily soluble in organic solvents and poorly soluble in water[3]. Its structural formula is shown in Figure 1.
As can be seen in Figure 1, the lycopene molecule contains multiple double bonds, so it is easily oxidized and unstable. Changes in light, O2, or pH can all lead to structural changes and oxidative degradation of lycopene[4].
1.2 β-carotene pigment
Beta-carotene is the most common natural pigment in nature, and is abundant in fruits and vegetables such as carrots, spinach and mangoes. It is a type of isoprene pigment. It is a dark red powder that is insoluble in water but soluble in benzene and chloroform. Iron ions, light, oxygen, etc. can all cause it to fade, so beta-carotene is not very stable [5]. Its structural formula is shown in Figure 2.
As can be seen in Figure 2, β-carotene pigment is highly unsaturated, containing many double bonds and branches, and is a good singlet oxygen quencher. The more conjugated double bonds, the redder the β-carotene [6].
1.3 Turmeric yellow pigment
Turmeric yellow pigment is a yellow pigment (with a fragrant aroma) that is mainly found in the tubers of turmeric and saffron. It belongs to the flavonoid class and mainly includes three active ingredients: curcumin, demethoxycurcumin and bisdemethoxycurcumin. The melting point is 179~182 °C, insoluble in water, lipophilic, soluble in glacial acetic acid, propylene glycol, ethyl acetate, alkaline solutions and 95% ethanol. It is easily discolored by iron ions and has poor light and heat stability [7]. Its structural formula is shown in Figure 3.
As can be seen from Figure 3, the curcuminoid molecule is highly reducible (contains double bonds, hydroxyl groups, and carbonyl groups), is prone to chemical reactions, and has good coloring power, especially for proteins. Zhu Jinshun et al. [8] studied the stability of the monomer curcumin: as the pH increases, the curcuminoid gradually changes from yellow (pH 4) to reddish brown (pH 10). Sucrose and maltose have a color-enhancing effect on curcumin; vitamin C, sodium ions, potassium ions and magnesium ions have no significant effect on curcumin.
1.4 Anthocyanins
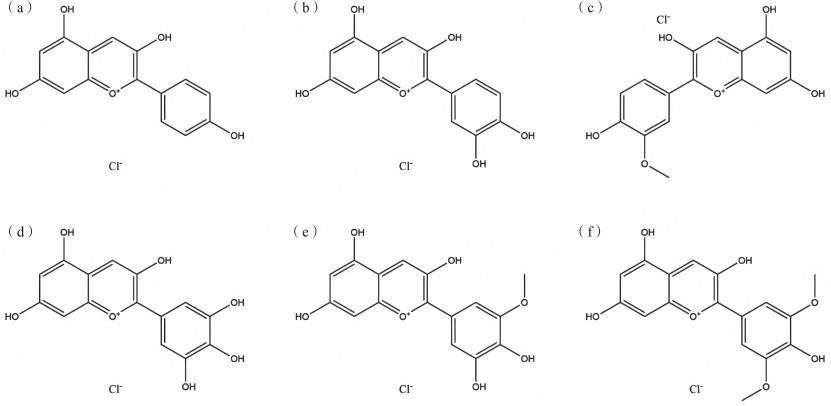
Anthocyanins (also known as anthocyanidins) are a type of flavonoid compound. They are water-soluble natural coloring agents that are easily soluble in ethanol but insoluble in vegetable oil. They are one of the main pigments that form the color of flower petals and fruits. It is mainly found in the skins of grapes, tomatoes and cherries, as well as in the flowers and fruits of plants such as strawberries and morning glories. Most red and purple fruits and vegetables contain anthocyanins[9]. The color of anthocyanins is related to pH (acid red alkali blue). The structural formula is shown in Figure 4.
As can be seen in Figure 4, the basic structural unit of anthocyanin is a 3,5,7-trihydroxy-2-phenylpyran cation. The number of hydroxyl groups, methylation and glycosylation positions and degrees, types of sugars, and the types and quantities of aromatic and fatty acids attached to anthocyanin also vary, which causes anthocyanin to appear in different colors in solution. More than 20 types of anthocyanins have been identified [10]. Anthocyanins are unstable and their stability is affected by pH, oxygen and metal ions. Anthocyanins with many hydroxyl groups in the structure are less stable than those with many methoxy groups [11].
1.5 Betalain
Beetroot pigments are mainly found in the flowers and fruits of plants, as well as in the vegetative tissues of most families in the Caryophyllales. They are also found in some higher fungi, such as Amanita muscaria [12]. They are a type of water-soluble nitrogen-containing pigment that is a derivative of pyridine. There are two main structures of beetroot pigments: the reddish-purple betalain and the yellow betaxanthin. It is poorly soluble in acetic acid and propylene glycol, and insoluble in ethanol, glycerin and fats. Currently, more than 50 betalains and more than 20 betaxanthins have been discovered [13]. According to their chemical structures, betalains can be divided into betaine, amaranthine, myrcianthine and decarboxylated types. Their structural formulas are shown in Figure 5.
As can be seen in Figure 5, the basic group of betalain pigments is alanine, the R group of betaxanthin is mainly amino acid (or amine), and the R group of betacyanin is mainly glycosyl. Betalains are soluble in water and appear reddish-purple. They are stable in the pH range of 4–7; when the pH is less than 4 or greater than 7, the color of the solution changes from red to purple; when the pH is greater than 10, the color of the solution quickly turns yellow (i.e., betaxanthin becomes betacyanin). Research has shown that betalains and anthocyanins are mutually exclusive (i.e., they cannot exist in the same plant at the same time) [14]. The stability of betalain is inversely proportional to its water activity. Ascorbic acid protects betalain [15].
1.6 Gardenia yellow pigment
Gardenia yellow pigment is mainly found in the fruits of Gardenia jasminoides, a member of the Rubiaceae family. It is one of the few water-soluble carotenoids in Natural Coloring. It is an isoprenoid pigment that is an orange-yellow powder (lemon yellow in an aqueous solution). Its main components are crocetin and crotonic acid. It is easily soluble in water, soluble in polar solvents such as ethanol and propylene glycol and other polar solvents, but is poorly soluble in non-polar solvents such as benzene and gasoline, and is insoluble in oil and fat. Its structural formula is shown in Figure 6. As can be seen in Figure 6, the parent nucleus of gardenia yellow pigment contains 7 conjugated double bonds, and different numbers of glucose can be attached to both ends (which greatly increases the polarity of gardenia yellow pigment). and therefore gardenia yellow is one of the few water-soluble carotenoids in nature. Its color is less affected by the pH of the environment (it remains yellow at pH 3–9; it is more stable than β-carotene at pH 4–6 or pH 8–11), and it is more light- and heat-resistant under neutral or alkaline conditions than under acidic conditions. In addition, gardenia yellow is easily absorbed by the human body, can be converted to VA in the human body, and has a strong coloring ability for proteins and starches. It is often used industrially to dye hydrophilic foods. Cu2+ and Fe3+ can cause it to darken, so it should be stored away from these substances.
1.7 Chlorophyll
Chlorophyll is a pyrrole derivative that plays a catalytic role in photosynthesis. Therefore, all organisms that carry out photosynthesis basically contain chlorophyll. Higher plants mainly contain two types of chlorophyll: a and b. The difference between them is a group on the pyrrole ring: chlorophyll a has a methyl group on the pyrrole ring, while chlorophyll b has a formyl group. This structural difference also causes them to have different colors (chlorophyll a is blue-green, while chlorophyll b is yellow-green). It is insoluble in water, but soluble in organic solvents such as ethanol and ether[16]. Its structural formula is shown in Figure 7.
As can be seen from Figure 7, the four pyrrole rings form a porphyrin ring. If the R group on the porphyrin ring is mainly methyl, it is a chlorophyll a molecule; if the R group is a formaldehyde group, it is a chlorophyll b molecule. The reason why the chlorophyll molecule is green is because the conjugated system formed by the single bond and double bond in the porphyrin ring absorbs some visible light. The chlorophyll molecule is unstable in its natural state, but its stability is greatly improved by replacing the magnesium ion in chlorophyll with ions such as copper, iron, and zinc [17-18].
1.8 Monascus red pigment
Monascus red pigment is a secondary metabolite of Monascus, which is a natural coloring prepared by fermenting rice, soybeans and other raw materials with Monascus. Its chemical structure can be divided into two parts: fatty acids and polyketones. Its melting point is 160 ~192 ℃, and it is soluble in water, ether, acetic acid, chloroform, hexane and other solvents. It has poor stability to light, temperature, acid and alkaline solutions, and is prone to fading. Its structural formula is shown in Figure 8.
As can be seen from Figure 8, the reason why monascus red pigment is unstable is that its molecular structure contains highly conjugated double bonds. Monascus red pigment is relatively stable in neutral environments, and is more stable in alkaline conditions (pH 9–11) than in acidic environments (pH 3–5). Since the double bond in the red yeast rice pigment molecule can be oxidized by Cu2+ and Fe3+, the color of its solution will change from bright red to reddish brown (and a reddish brown precipitate will form). Therefore, iron and copper utensils should be avoided when storing red yeast rice pigment.
1.9 Paprika red pigment
Paprika red (also known as paprika or capsicum red) is mainly derived from the pericarp of red peppers in the Solanaceae family. It is a carotenoid pigment with a pungent pepper aroma. It is a kind of isoprene pigment, and its main components are paprika red and capsicum rubrum. The melting point is about 175 °C, it is insoluble in water and glycerin, but soluble in polar organic solvents. It turns blue when reacting with concentrated inorganic acids. The structural formula is shown in Figure 9.
As can be seen in Figure 9, paprika red contains multiple unsaturated double bonds, which will accelerate its oxidative decomposition and discoloration under aerobic conditions. It can form a precipitate with Pb3+, and Cu2+ and Fe3+ can also cause it to discolor (but K+, Ca2+, Na+, Mg2+, and Zn2+ have no effect on its stability, and these metal ions can be used as additives together with paprika red). In addition, paprika red is highly resistant to heat (25–70 °C) and acids (pH 3–12), but less so to light (stable to visible light, but easily discolored by UV light).
1.10 Other
In addition to the natural pigments that are currently used in the processing industry, as described above, some new natural coloring have been discovered in recent years, such as perilla pigment extracted from the leaves of perilla plants in the mint family; red cabbage pigment extracted from the leaves of red cabbage; sorghum pigment extracted from the husks of sorghum plants in the grass family; and red rice pigment extracted from red rice. In addition, natural coloring pigments such as cochineal pigment, lobster red pigment and crab shell pigment can also be extracted from animals[19].
2 Separation and purification methods for natural coloring
The main separation and purification methods currently used for natural coloring are: supercritical fluid extraction, microwave-assisted extraction, molecular distillation, solvent extraction, enzymatic reaction, etc.
2.1 Supercritical fluid extraction
Supercritical fluid extraction (SFE) is a technology that involves the use of supercritical fluids. When a substance is at a specific temperature and pressure (8–50 MPa; 35–80 °C), the interface between the gas and liquid phases disappears, and the state is called the supercritical state. The fluid in this state is called a supercritical fluid (SCF). SCF), SCF is a high-density fluid with the advantages of both gas and liquid. It has the density and solubility of a liquid and the low viscosity and high permeability of a gas. The solubility of SCF changes with temperature and pressure to achieve the purpose of separating extracts. Since the supercritical temperature of CO2 (31 °C) is close to room temperature and non-toxic and non-polluting, it does not corrode equipment and is often used as a supercritical fluid[20]. The optimal process conditions for supercritical fluid extraction of different types of natural coloring vary, generally in the range of 10~50 MPa, 31~80 °C, and 3~20 h[21-22].
2.2 Microwave-assisted extraction
Microwave-assisted extraction, also known as microwave extraction (MAE), uses a dipole that rotates at high speed under the action of microwaves, constantly changing between positive and negative poles to generate eddy currents and friction as well as ion conduction. A large amount of heat in a short period of time causes the hydrogen bonds between cell molecules to break, which in turn causes the cell membrane structure to break. The temperature rise caused by friction and collisions leads to the rupture of the cell wall. all of which accelerates the diffusion of the Natural Coloring inside the cell to the extraction agent.
Microwave extraction also has very good selectivity, selectively heating substances with different dielectric properties. Heating is inversely proportional to the dielectric constant and directly proportional to polarity. The advantage of microwave-assisted extraction of Natural Coloring is that the high-frequency fluctuations of microwaves can accelerate the dissolution of Natural Coloring in the extraction agent, which improves the extraction efficiency while reducing the extraction time. Microwave also has heating function, heat energy can extract several components at the same time in a very short time, and microwave-assisted extraction can greatly reduce the amount of extraction solvent, not only save energy, but also reduce pollution, and the results are also reproducible.
2.3 Molecular distillation technology
Molecular distillation (MD) is also known as short-path distillation. It is a high-tech method of separation that uses the principle that the mean free path of molecular motion (the average distance between two consecutive collisions of a gas molecule) of different substances is different. First, the heating device heats the mixture to be separated. When the molecules gain sufficient energy, they escape from the evaporation surface. The mean free path of light molecules is larger than that of heavy molecules. A condensation surface is set between the free paths of heavy and light molecules. Since heavy molecules cannot reach the condensation surface and return to the evaporation surface, they maintain the original dynamic balance and do not escape.
Light molecules can reach the condensation surface and condense continuously, destroying the dynamic balance of light molecules and separating the light molecules. The molecular distillation process of natural coloring is mainly divided into five steps: (1) Natural coloring molecules diffuse from the liquid phase to the evaporation surface. (2) Natural coloring molecules evaporate freely from the evaporation surface. (3) Natural coloring molecules fly from the evaporation surface to the condensation surface. (4) Natural Coloring molecules condense on the condensing surface. (5) Collect the distillate and residue. The advantages of molecular distillation are low operating temperatures, low pressure, high separation efficiency, and separation of substances without contamination. This not only reduces separation costs, but also protects the structure of the natural coloring from damage.
2.4 Solvent extraction method
Solvent extraction of Natural Coloring can be divided into hot extraction and cold extraction. The solvent with high solubility for the Natural Coloring and low solubility for the components that do not need to be dissolved is selected according to the polarity of the extracted Natural Coloring, the physicochemical properties of the coexisting impurities, and the principle of like dissolves like. The solvent enters the sample cells through osmosis and diffusion, dissolving the Natural Coloring and a small amount of impurities to form a concentration difference between the inside and outside of the cells. The concentrated solution in the cells continuously diffuses outward to achieve the purpose of extracting the Natural Coloring. The advantages of the solvent extraction method are: the solvent is cheap, the operation is simple, and the extraction rate is high. The disadvantages are: the extracted Natural Coloring has poor quality, low purity, an unpleasant odor, solvent residue, etc.
2.5 Enzyme extraction method
Natural Coloring is generally found inside plant cells. When extracting Natural Coloring from plants, it is necessary to penetrate not only the cell membrane but also the plant cell wall, which greatly reduces the extraction rate of Natural Coloring. If the plant cell wall can be removed, it will greatly increase the extraction rate of Natural Coloring. The plant cell wall is composed of cellulose and pectin, and cellulase can dissolve the plant cell wall to facilitate the extraction of ingredients. Yu Huajuan et al. [23] showed that compared with the water immersion method, enzymatic extraction increased by 9.40% to 13.35%. Natural coloring and specific enzymatic reactions produce the desired colors. For example, gardenia yellow pigment gardenia glycoside can polymerize with primary amino acids (α-amino acids) under the action of β-glucosidase or β-galactosidase to produce blue pigments.
There are many methods for isolating and purifying natural coloring, but the current extraction rates are not ideal. In addition to the above methods, there are also chromatographic separation, column chromatography, alkaline extraction and membrane separation methods [24-26].
3 Overview of natural coloring applications
3.1 Natural coloring in food
As the saying goes, food is the most important thing to people. People often describe a good dish as being “colorful, fragrant and delicious”. People's first impression of food is its color. When you fry carrots, you can see that the oil turns orange, and when you blanch amaranth, it turns red. Natural Coloring not only gives food an attractive color (such as colorful dumplings, glutinous rice balls, wheat, corn, fruit-filled mooncakes, corn buns, taro buns, and colorful little animals on various cakes, etc.), but also a qualitative leap in nutritional value, giving consumers a good sensory experience and a strong desire to buy.

Although natural coloring is used in small amounts, it has a significant impact on the quality of the food[27-30]. Natural coloring not only enhances the flavor of the food, but also has the effect of antibacterial, bacteriostatic, and extending the shelf life of the product. Since it cannot be eaten alone as food, it is mainly used in condiments such as soy sauce, chili sauce, pickles, and chili oil. For example, red yeast rice red pigment can increase the redness index of soy sauce, can be used to improve the color of soy sauce, which is used for seasoning and braising, so that it does not turn black; gardenia yellow pigment can make the golden yellow color of soy sauce more obvious; radish red pigment and lac dye red pigment are used to color acidic products such as chili sauce; safflower yellow pigment can be used to color orange-yellow products such as sweet oranges, pineapples, and mangoes; β-carotene and turmeric yellow pigment can be used to enhance the color and nutrition of chicken essence; paprika red pigment is used for oil spraying and coloring of biscuits; gardenia yellow pigment is used for coloring instant noodles; sodium copper chlorophyllin and tomato red pigment are used for coloring vegetable-type dried noodles; curcumin is used for decorating cakes and coloring the fillings of mooncakes; and red rice red pigment is used for coloring baked goods.
3.2 Natural Coloring in medical and healthcare applications
In recent years, there have been frequent reports of a high incidence of various cancers and tumors. While people are afraid of cancer, they are also eager to find out the causes and preventive measures. Many medical studies and clinical trials have shown that excess free radicals (ROS) in the human body will rob electrons everywhere in the body. If the electrons of protein molecules are captured by ROS, the proteins will be alkylated by branched chain linkages, and the protein molecules will be distorted, leading to the occurrence of cancer. Studies have shown that most natural colorings have the effect of quenching singlet oxygen in the human body, removing free radicals, enhancing the body's immune system and preventing the occurrence of cancer [31].
Chlorophyll and its derivatives have a wide range of medicinal and health benefits. The chlorophyll molecule is similar in structure to the human hemoglobin molecule. Nobel laureates Dr. Richard Willstatter and Dr. Hans Fischer discovered that chlorophyll has hematopoietic function. Drinking chlorophyll can greatly help the blood recovery of women who have just given birth and people who have lost blood accidentally. Chlorophyll derivatives: chlorophyll zinc and sodium salts are effective in clinical medicine for treating zinc deficiency in young children, skin damage, and gastrointestinal ulcers. Chlorophyll and its derivatives can also be taken orally to aid healing. In addition, chlorophyll and its derivatives can be used to assist in the treatment of infectious hepatitis, hemorrhoids, and leukemia. The amount of lycopene in the body is related to the length of life[32 -36]. The human body cannot synthesize it, so it must be ingested from the outside. It not only regulates cholesterol metabolism to prevent cardiovascular disease, but also has a special effect on male infertility.
Clinical controlled trials by German doctors have shown that middle-aged and elderly men who supplement with sufficient lycopene every day have an 80% lower incidence of prostate cancer than those who lack lycopene [37]. Middle-aged and elderly women who regularly supplement with lycopene can greatly reduce the incidence of gynaecological diseases such as breast cancer and uterine cancer, and also have a certain preventive effect on osteoporosis. Beta-carotene can inhibit lipid peroxidation, improve immunity, enhance insulin sensitivity, thereby protecting eyesight and reducing the incidence of cancer and diabetes [38]; curcumin has anti-inflammatory, anticoagulant, anti-infective and anti-oxidative modification of low-density lipoprotein effects, can lower blood lipids and prevent the formation of age spots and atherosclerosis; zeaxanthin can be converted to VA in the human body, so it helps to protect and restore eyesight and also enhances the body's immune system [39]; grape skin red pigment has the effect of preventing coronary heart disease and atherosclerosis; anthocyanins have the functions of anti-inflammatory, anti-tumor, and improving eyesight, while also inhibiting the oxidation of lipoproteins and platelet aggregation, so they can effectively prevent the occurrence of cancer. Perilla pigment has the functions of detoxification, dispersing cold, regulating qi and stomach. Gardenia yellow pigment extracted from gardenia fruit has the ability to reduce inflammation, relieve fever, promote bile flow and resist oxidation[40].
3.3 Natural Coloring in Cosmetics
Gardenia yellow pigment, β-carotene, safflower yellow pigment, caramel, cocoa pigments and other natural colorings have strong free radical scavenging and singlet oxygen quenching abilities, and are often used in sunscreens, skin care products and other types of cosmetics. This is because the removal of free radicals can effectively prevent skin cells from being damaged by free radicals, reducing the production of wrinkles and freckles; quenching singlet oxygen can protect the skin from ultraviolet damage, prevent photoaging of the skin, and prevent skin cancer.
Natural cosmetics provide healthy ingredients for the skin while also reducing the burden on the skin, greatly improving the respiration of skin cells and the water exchange rate. The great advantage of natural cosmetics is that they are antibacterial and anti-inflammatory without irritating the skin [41-42]. For example, caramel coloring is very cost-effective, but it is also very stable and does not change under conditions such as light or high temperatures. It is also almost unaffected by changes in pH. Caramel coloring is not only bright and colorful, but also fragrant and easy to apply. Rubiachin is extracted from the traditional Chinese herb Rubia cordifolia, and has a good spot-removing effect; lycopene not only gives a bright red color and makes cosmetics appear attractive, but also has strong antioxidant properties that can extend the shelf life of cosmetics. It can also be used as a coloring agent in cosmetics such as lip balm and lipstick; cocoa pigment is heat-resistant and has good coloring properties. It can moisturize and hydrate the skin and delay skin aging. Natural Coloring can also prevent and treat various skin diseases [43-45]. With a deeper understanding of skin metabolism, the application of Natural Coloring in cosmetics will become more extensive.

3.4 Application of Natural Coloring in the dyeing industry
Many synthetic dyes can irritate the skin and cause skin allergies [46]. Direct dyes (which directly dye materials such as fibers without the need for other chemical dyeing aids), acid dyes (which can be dyed in an acidic medium) and disperse dyes (which are non-ionic dyes with poor water solubility) can also cause skin diseases and induce cancer. Natural Coloring dyes are mostly derived from animals, plants and microorganisms, and are highly safe, harmless, non-polluting and have unique and exquisite hues. Natural coloring has a long history of use in the dyeing industry. In the “Qimin Yao Shu” (The Essential Techniques for Common People) written by Jia Sixiao at the end of the Northern Song Dynasty, there is a record of people extracting natural pigments from plants as dyes. Because natural coloring dyes have many hydroxyl groups (which dye fabrics via van der Waals forces and hydrogen bonds) and are therefore highly hydrophilic, and synthetic fibers are highly hydrophobic, mordant (alum, copper sulfate, potassium dichromate, etc.) is needed to fix the color during dyeing.
This is because metal ions can form complexes with both the hydroxyl groups in the Natural Coloring dye and the hydroxyl groups in the fiber. The metal ions can link the dye molecules to the fiber, improving the dye uptake and dye fastness. Turmeric pigments can be used to dye cotton in a variety of colors through acid soaking and mordanting, and the wash fastness is very high. Catechins (polyphenols) in tea can be used as a mordant with copper sulfate to dye cotton and jute brown. Shellac, turmeric and onion pigments can be used to dye polyester fabrics. Onion dye cannot color polyester under normal pressure, but turmeric and shellac dyes can dye light colors; chlorophyll, cocoa pigments, gardenia yellow Natural Coloring can also be used to dye cellulose; Natural Coloring can be dyed at high temperatures and pressures by treating with various mordants (alum has a high dyeing rate, and copper sulfate has good light fastness). The high pressure method is more effective than the atmospheric pressure method [47-50].
4 Natural Coloring Problems in research and development and solutions
4.1 Problems
There are currently more than 80 known natural colorings [51]. Most natural pigments are non-toxic, but gambier is highly toxic. Therefore, “natural” is not equivalent to “safe,” and the safety of natural colorings should not be ignored. There are still some problems in the research and development of natural coloring: extensive but not in-depth research; delayed toxicological evaluation; poor stability; low extraction rate and purity; and a lack of comparability of research results on natural coloring.
4.2 Solutions
The following solutions can be adopted to address the above problems in the application of natural coloring: (1) conduct in-depth research on the structure, physiology and pharmacological health benefits of natural coloring; (2) Strictly regulate and approve newly developed natural colorings, and comprehensively evaluate their safety, such as chemical structure, stability, and toxicity in animal experiments, and establish corresponding ADI values (maximum allowable daily intake per person) [52]. (3) The stability of natural coloring can be greatly improved by combining different pigments, ion exchange, and adding stabilizers. For example, the stability of betalain is greatly improved after it is combined with tea pigments, and the stability of quinone pigments is greatly improved after alum is used as a stabilizer [53]. The chlorophyllin is very stable after copper replaces the magnesium in the chlorophyll and then a sodium or potassium salt is made. (4) The use of callus tissue and microorganisms to produce natural coloring not only expands the sources of natural coloring and increases the yield of natural coloring, but also reduces the restrictions of natural coloring on the environment, seasons, and species. For example, beet red pigment is produced from beet callus tissue, anthocyanins are produced by culturing grape cell suspensions, and saffron pigments can be produced by culturing saffron cells [54]. (5) Only after the isolation and purification of the same Natural Coloring from multiple sources, and systematic and comprehensive research and comparison of its structure, function and biological effects, will people have a deeper understanding of Natural Coloring.
5 Conclusion and outlook
In summary, Natural Coloring has superior properties that cannot be matched by chemically synthesized pigments, and its proportion in the pigment industry is increasing. With the deepening of research on Natural Coloring, the improvement of environmental awareness, and the development of fields such as food toxicology evaluation, Natural Coloring will replace chemically synthesized pigments, which have stronger toxic side effects, and become the new direction for the development of the pigment industry in the future.
However, the main reason for the current stagnation of research on natural coloring is that the components of natural coloring are complex and difficult to completely separate, purify and identify, which has led to a lack of understanding of the structure, properties, physiological activity and safety of natural coloring components. Ultimately, this is because the current separation and purification techniques are still immature. At this stage, efforts should be focused on the following areas: first, improving the extraction process and developing efficient and economical extraction and separation techniques. Second, improving the comprehensive utilization rate of by-products and increasing the added value of products. Third, developing better solutions to the shortcomings of natural pigments. Fourth, expanding the application fields of natural coloring. It is believed that in the near future, natural pigments will be more widely used in food, medicine and health care, printing and dyeing, cosmetics and other fields.
Reference:
[1] Eleonora M B,Daniel R C,Leif H,et al. Quenching of excited states of red-pigment zinc protoporphyrin IX by hemin and natural Reductors in dry-cured hams[J]. European Food Research and Technol- ogy,2011,232:343-349.
[2] Caro Y,Anamale L,Fouillaud M,et al. Natural hydroxy anthraqu -inoid pigments as potent food grade colorants: an overview [J]. Natural Products and Bioprospecting,2012,2(5):174-193.
[3] Ho K K H Y ,Ferruzzi M G ,Liceaga A M,et al. Microwave assisted extraction of lycopene in tomato peels: effect of extraction conditions on all-trans and cis-isomer yields [J]. LWT-Food Science and Technology,2015,62 (1):160 - 168.
[4] Kujawska M ,Ewertowska M ,Adamska T,et al. Antioxidant effect of lycopene -enriched tomato paste on N-nitro-sodiethylamine induced oxidative stress in rats [J]. Journal Physiol Biochem,2014,70(4):981-990.
[5] Frengova G I,Beshkova D M. Carotenoids from rhodotorula and phaffia:yeasts of biotechno-logical importance [J]. Industrial Microbiol Biotechnol, 2009,36(2):163-180.
[6] Yan Ru-Long,Luo Jia,Wang Chuan-Xin,et al. Cu (I) -catalyzed synthesis of polysubstituted pyrroles from dialkylethy lenedicarboxylates and β-Enamino ketones or esters in the presence of O2[J]. Journal of Organic Chemistry,2010,75 (15):5395-5397.
[7] Subudhi U,Chainy G B. Curcumin and vitamin E modulate hepatic antioxidant gene express - ion in PTU -induced hypothyroid rats [J]. Molecular Biolgy Reports,2012,39(11):9849 -9861.
[8] Zhu Jinshun, He Fang. Extraction process and pigment stability analysis of turmeric yellow pigment [J]. China Fiber Inspection, 2015 (1): 86-88.
[9] Hirth M ,Preiβ R ,Mayer -Miebach E,et al.Influence of HTST extrusion cooking process parameters on the stability of anthocyanins,procyanidins and hydroxycinnamic acids as the main bioactive chokeberry polyphenols [J]. LWT -Food Science and Technology,2015,62 (1):511-516 .
[10] Nems A, Peksa A, Kucharska A Z,et al. Anthocyanin and antioxidant activity of snacks with coloured potato [J]. Food Chemistry, 2015,172:175-182.
[11] Chandrasekhar J,Madhusudhan M C,Raghava - rao K S M S. Extration of anthocyanins from red cabbage and purification using adsorpion[J]. Food and Bioproducts Processing,2012,90: 615-623.
[12] Huang Huan, Hu Huixia, Jiang Lianhe, et al. Research progress on plant beetroot pigments [J]. Bulletin of Biology, 2014, 49(8): 1-4.
[13] Chauhan S P,Sheth N R,Rathod I S,et al.Analysis of betalains from fruits of opuntia species [J]. Phytochemistry Reviews,2013 (12):35-45.
[14] Ravichandran K,Saw N M M T,Mohdaly A A A,et al. Impact of processing of red beet on betalain content and antioxidant activity[J]. Food Research International,2013,50(2):670- 675.
[15] Cao Jiliang, Cao Yi, Zhang Chengyu, et al. Discussion on the stability of betalain [J]. Dyeing and Finishing Technology, 2014, 36(3): 28-31.
[16] Liu Weibing,Jiang Huanfeng,Bin Liang. One potsilvercatalyzed and PIDA -mediated seqential reaction:synthesis of poly -substituted pyrroles directly from alkynoates and amines[J]. Organic Letters,2010,12(2):312-315 .
[17] Sukhendu M,Srijit B,Umasis S J N. Iron (III)catalyzed four -component coupling reaction of 1,3-dicarb-only-compounds,amines,aldehydes, and nitroalkanes:a simple and direct synthesis of functionalized pyrroles[J]. Journal of Organic Chemistry,2010,75(5):1674-1683.
[18] Jessica G G,Sarah J P Y,Nathan R B . Synthesis of unsymmetrical 3,4 -diaryl -3 - pyrrolin-2-ones utilizing pyrrole weinreb amides [J]. Journal of Organic Chemistry Chem,2011,76(20):8203-8214.
[19] Yang Shuangchun, Li Chunyu, Pan Yi. Research progress of microbial pigments in the food industry [J]. Food Research and Development, 2014, 35(1): 114-117.
[20] Zhang Y. Comparison of supercritical extraction and organic solvent extraction of capsanthin [J]. Chinese condiments, 2013 (4): 101-103.
[21] M -Sanchez W D. Supercritical fluid extraction of carotenoids and cholophylla from nannoch loropsis gaditana [J]. Journal of Food Engineering,2005, 66:245-251.
[22] Liang M T,Yang C H,Li S T , et al. Antibacterial and antioxidant properties of ramulus cinnamomi using supercritical CO2 extraction [J]. European Food Research and Technology,2008,227(5):1387-1396.
[23] Yu Huajuan, Sun Zhida, Xie Bijun. Study on the process of enzyme-assisted extraction of lotus flower anthocyanins and their oxidation activity [J]. Natural Product Research and Development, 2010, 22 (10): 154-158.
[24] Suo Q L,He W Z. Micronization of the natural pigment bixin by the SEDS process through prefilming atomization[J]. Powder Technology, 2005,154:110-115.
[25]Chen Dan,Wu Zanmin. Study on extraction puri- fication process of capsicum red pigment[J]. Journal of Agricultural Science,2009,1(20):94- 100.
[26] Xin A,Xuefeng L,Zouqing Z,et al. RAPD teachnique used todeter-minthe purity of hybrid hot pepper seeds[J]. Hunan Agricultural Science and Technology Newsletter,2000(1):119-123.
[27] Munawar N,Jamil H M T H. The islamic perspective approach on plant pigments as natural food colourants[J]. Procedia Social and Behavioral Sciences,2014,121:193-203.
[28] Spoerle. Application of natural coloring in compound condiment foods [J]. Chinese Condiments, 2011, 36(2): 97-101.
[29] Miao X. Research status and development prospects of edible natural coloring [J]. Chemical Industry Management, 2013(5): 5-7.
[30] Bridle P,Timberlake C F. Anthocyanins as natural food colours selected aspects [J]. Food Chemistry,1997,58(2):103-109 .
[31] A ssous M T M,Abdel H M M,Medany G M. Evaluation of red pigment extracted from purple carrots and its utilization as antioxidant and natural food colorants[J]. Annals of Agricultural Sciences,2014,59(1):1-7.
[32] Bao Huayin. Research progress in the pharmacological effects of lycopene in the past five years [J]. Food Research and Development, 2014, 35 (19): 145-147.
[33]Tamilselvan P,Langeswaran K,Vijayaprakash S,et al. Efficiency of lycopene against reproductive and developmental toxicity of bisp -henol a in male sprague dawley rats [J]. Biomedicine Preventive Nutrition,2014,4(4):491-498.
[34] Chen W ,Lu Y ,Wu J ,et al. Beta -elemene inhibits melanoma growth and metastasis via suppressing vascular endothelial growth factor mediated angiogenesis [J]. Cancer Chemother Pharmacol,2011,67(4):799 .
[35] Lopes L B,Vandewall H,Li H T,et al. Topical delivery of lycopene using microemulsions: enhanced skin penetration and tissue antioxidant activity [J].Journal of Pharmaceutical Sciences,2010,99(3):1346-1357.
[36] Zhang Yanmei, Huang Zeqing, Gao Haoran, et al. Regulation of lycopene on perioperative immune function in lung cancer patients [J]. Journal of Liaoning Medical College, 2011, 32(1): 35-37.
[37] Chen Liping,He Shuying,Zheng Heng,et al. Effectsand mechanisms of lycopene on the proliferation of vascular smooth muscle cells [J]. Chinese Journal of Natural Medicines, 2010(3):218-222 .
[38]Van P G,Goldbohm R A. Epidemiologic evidence for beta caroteneand cancer prevention[J].The American Journal of Clinical Nutrition,1995,62: 1393-1402.
[39] Du Zehua, Ren Jiaoyan. Research progress on the extraction process and biological activity of zeaxanthin [J]. China Food Additives, 2014 (2): 214-219.
[40] Zhou Tanyang, Luo Furong, Bai Bin. Research progress on the bio-pharmacological activity of grape seed proanthocyanidins [J]. Harbin Medical University Journal, 2012, 46(1): 94-96.
[41] Mcardle F,Rhodes L F,Parslew R A G,et al.Effects of oral vitamin E and β -carotene supplementation on ultra viole tradiation induced oxidative stress in human skin[J].The American Journal of Clinical Nutrition,2004,80:1270-1275.
[42] Wulf H C,Sandby-Moller J,Kobayasi T,et al. Skin aging and natural photoprotection [J]. Micron,2004,35(3):185-191.
[43] Yeh S L,Hu M L,Huang C S. Lycopene enhances UVA -induced DNA damage and expression of heme oxygenase -1 in cultured mouse embryo fibroblasts [J]. European Journal of Nutrition, 2005,44(6):365-370 .
[44] Darvin M,Patzelt A,Gehse S,et al. Cutaneous concentration of lycopene correlates significantly with the roughness of the skin [J]. European Journal of Pharmaceutics and Biopharmaceutics, 2008,69(3):943-947 .
[45] Blanch G P,Castillo M L,Mar -Caja M,et al. Stabilization of all -trans lycopene from tomato by encapsulation using cyclo-dextrins[J]. Food Chemistry,2007,105(4):1335-1341 .
[46] Yang Jianjun, Cui Yan. Dyeing technology and mordant method of natural dyes [J]. Journal of Tonghua Normal University: Humanities and Social Sciences, 2014, 35 (6): 37-43.
[47] Bulut M O,Akar E. Ecological dyeing with some plant pulps on woolen yarn and cationized cotton fabric [J]. Journal of Cleaner Production,2012,32:1-9 .
[48] Ibrahim N A,El-Gamal A R,Gouda M,et al. A new approach for natural dyeing and functional finishing of cotton cellulose [J]. Carbohydrate Polymers,2010,82(4):1205-1211.
[49] Sava V M,Yang S M,Hong M Y,et al. Isolation and characterization of melanic pigments derived from tea and poly phenols [J]. Food Chemistry, 2001,73(2):177-184.
[50]Kamel M M,El-Shishtawy R M,Yussef B M,et al. Ultrasonic assisted dyeing Ⅲ.dyeing of wool with lac as a natural dye [J]. Dyes and Pigments,2005,65(2):103-110.
[51]Caro Y,Anamale L,Fouillaud M,et al. Natural hydroxy anthraquinoid pigments as potent food grade colorants:an overview [J]. Natural Products and Bioprospecting,2012,2(5): 174-193.
[52] Cheng Li. Characteristics, application, safety evaluation and safety control of natural food coloring [J]. Food Science, 2012, 33(23): 399-404.
[53] Ali N F,El -Mohamedy R S R. Eco -friendly and protective ntaural dye from red prickly pearly pear (Opuntia Lasiacanthapfeiffer) plant [J]. Journal of Cleaner Production,2003,11 (5):499-509 .
[54] Marie -Rose V C,Martine C B,Francois C,et al. Spectroscopic characterization of crocetin derivatives from crocus sativus and gardenia jasminoides[J]. Journal Agriculture Food Chemist ry,1997,45:1055-1061.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese







