What Is the Benefit of Beta Carotene Powder?
β-carotene is used as a coloring agent and vitamin-type functional feed additive. It has strong antioxidant capacity and can reduce the production of lipid oxidation by scavenging free radicals in the body. It has the effect of promoting the healthy growth of animals, reducing feeding costs and improving the value of animal products [1]. This paper reviews the physical and chemical properties and sources of β-carotene, its absorption and metabolism in animals, its main physiological functions and the progress of its application in animal production, providing a reference for the application and promotion of β-carotene in modern healthy aquaculture production.
1 Physical and chemical properties of β-carotene
β-Carotene is an orange-yellow fat-soluble compound. As a key precursor of retinol, it is an important regulator of body fat reserves. Beta-carotene products are mostly red, brownish red, or purple crystals or crystalline powders. They are insoluble in water, slightly soluble in ethanol and ether, and easily soluble in organic solvents such as chloroform and benzene. They have a melting point of 176-180°C and are easily degraded by heat, light, and oxygen.
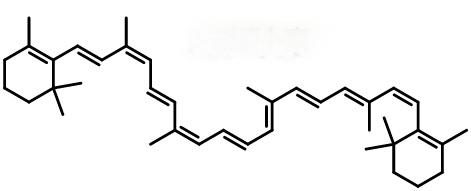
The molecular formula of β-carotene is C40H56, with a molecular weight of about 537. It contains 15 conjugated unsaturated double bonds and two β-ionone rings at each end (Figure 1). The common β-carotene structures in nature are all-trans, 9-cis, 13-cis and 15-cis, among which all-trans β-carotene is easily absorbed and utilized by animals [2].
2 Sources of β-carotene
β-carotene is mainly obtained naturally and through chemical synthesis. Natural sources of β-carotene are mainly extracted from plants, microorganisms, algae, and synthesized by microorganisms such as yeast. Chemical synthesis generally uses β-ionone as the starting material. Currently, more than 90% of β-carotene products are of chemical synthesis origin [3]. The use of solvents and by-products in the chemical synthesis of β-carotene products can affect human safety, while β-carotene products from natural sources have better medical and nutritional effects. However, no safety issues have been found in toxicity studies of β-carotene products from different sources [4].
Due to the powerful nutritional and medical bioactivity of β-carotene, research on industrialization technology for products of natural origin has gradually attracted attention, mainly focusing on the extraction and biosynthesis of Dunaliella salina. Microbial fermentation is a popular β-carotene biosynthesis technology in recent years, mainly using genetically engineered bacteria such as Brevibacterium linens [5] and Rhodafuva yeasts [6], but it has not yet been industrialized.
In China's feed additive catalog, β-carotene is clearly defined as a vitamin and vitamin-like feed additive product, and national standards for β-carotene feed additives have been formulated for natural fermentation and chemical synthesis: GB/T 19370-2003 and GB 7300.901-2019. These standards clearly require that chemically synthesized β-carotene be used as a coloring agent.

3 Absorption and metabolism of β-carotene in the body
3.1 Absorption of β-carotene in the body
In nature, β-carotene is widely distributed in green leafy vegetables, yellow and orange vegetables and orange fruits. It is also stored in bacteria and animals, but animals and humans cannot synthesize it themselves and must obtain it from the outside. In animals, the intestine is the main organ for the digestion and absorption of β-carotene, while the liver and spleen are the main storage organs. β-Carotene is mainly absorbed and converted in the proximal and middle small intestine [7]. β-Carotene is absorbed in the small intestine of animals in the form of intact chylomicrons and is distributed to other organs of the body. Its absorption in the intestine is affected by factors such as the concentration of intestinal bile salts and the particle size of chylomicrons [8].
3.2 β-Carotene metabolism in the body
There are two main metabolic pathways for β-carotene after absorption [9] (Figure 2), which are conversion to vitamin A and breakdown to produce retinol or retinoic acid. β-Carotene is converted to vitamin A by the action of 15, 15'-oxygenase and retinal reductase, and exerts its nutritional role as vitamin A. Among these, β-carotene 15, 15'-monooxygenase (BCMO1) and β-carotene 9', 10'-dioxygenase ( BCDO2) are the main enzymes in adult tissues that cleave β-carotene to produce vitamin A.
Under the action of BCMO1, the 15,15' double bond of β-carotene is cleaved symmetrically by oxidation to produce 2 molecules of retinal. Retinal can be converted to trans-retinoic acid by the action of aldehyde dehydrogenase or retinal dehydrogenase, which is the biologically active form of vitamin A. BCDO2 can catalyze the cleavage of β-carotene by non-centrosymmetric cleavage to 10'-apocarotenal and β-ionone, of which 10'-apocarotenal can be converted to 1 molecule of retinal. Turner et al. [10] found that After being absorbed by the small intestinal epithelial cells, β-carotene is converted to retinal by BCMO1, and then reversibly converted to retinol in the liver by retinal reductase and stored. After being stored in the liver, retinol is hydrolyzed and combined with retinol-binding protein for transport to target cells. Unabsorbed β-carotene is excreted in the feces.
4 Main physiological functions of β-carotene
4.1 Synthesis of vitamin A
The multiple unstable conjugated double bonds in β-carotene are easily oxidized and isomerized in the presence of heat, oxygen, and light, producing various degradation products such as phytohormones, aroma substances, and vitamin A. In 1930, Moore [11] confirmed that β-carotene is a precursor of vitamin A by supplementing vitamin A-deficient rats with β-carotene, which significantly increased the vitamin A levels in the body. proving that β-carotene is a precursor of vitamin A. In humans and mice, β-carotene metabolic enzymes BCMO1 and BCDO2 are expressed in various tissues of the body, including the liver, fat, placenta and embryo [12], indicating that β-carotene can be used as a source of vitamin A in various parts of the body.
4.2 Antioxidant function
The structure of β-carotene contains multiple conjugated polyene double bonds, which have strong free radical scavenging capacity and antioxidant activity. They can reduce the production of lipid oxidation in cells and alleviate the adverse effects of oxidative stress [13]. Zhang Xianglun et al. [14] found that the β-carotene and serum antioxidant properties of beef serum were positively correlated with the amount of dietary β-carotene added, while the serum vitamin A content was not significantly correlated with the amount of dietary β-carotene added or serum antioxidant properties, indicating that β-carotene may directly affect the serum antioxidant properties of beef cattle, rather than vitamin A, which is a product of catabolism.
The possible antioxidant mechanisms of β-carotene are currently being analyzed: ① The double bond structure binds to free radicals through mechanisms such as hydrogen atom transfer, free radical addition, and electron transfer [15], exerting a free radical scavenging effect. ② The electronic arrangement of the β-carotene nucleus is similar to that of lycopene, and it may exert a quenching effect on singlet oxygen due to energy transfer by electron exchange [16]. ③ β-carotene can promote the antioxidant process by changing the activity of antioxidant enzymes such as glutathione-S-transferase, inhibiting the expression of NADPH oxidase subunits and increasing the expression/activity of antioxidant enzymes [17]. Zhou Tong et al. [18] found that in a model of intermittent hypoxia-induced cognitive impairment in rats, feeding beta-carotene can reduce neuronal cell apoptosis and restore cognitive function in rats by scavenging reactive oxygen species (ROS). Beta-carotene can increase the activity of glutathione peroxidase (GSH-Px) in the liver and serum [19]. Further in-depth research has found that β-carotene can maintain the body's redox balance by upregulating Nrf2 mRNA expression, increasing the mRNA expression of antioxidant enzymes, regulating Superoxide Dismutase (SOD) activity, and reducing Malondialdehyde (MDA) levels [20-21].
4.3 Anti-inflammatory function
Beta-carotene exerts its anti-inflammatory function mainly by activating signal pathways such as MAPK and NF-κB in the body and regulating the expression of cytokines. Qiao Dong et al. [22] administered β-carotene to mice by gavage and intraperitoneal injection and found that β-carotene could promote the production of inflammatory cytokines. The intensity of the effect on cytokines varied depending on the pathway, and it was speculated that the immunomodulatory mechanism of β-carotene in the body may be related to signal pathways such as NF-κB and JAK-STAT.
Li et al. [23] investigated the effect of β-carotene on the activation of the JAK2/STAT3, MAPK and NF-κB signaling pathways in lipopolysaccharide-induced RAW264.7 cells and peritoneal macrophages. The results showed that β-carotene significantly inhibited the release of IL-1β, IL-6 and TNF-α release and their mRNA expression, and the LPS-induced activation of JAK2/STAT3, IκB/NF-κBP65, and JNK/p38 MAPK signaling was significantly and dose-dependently reduced by β-carotene. This indicates that β-carotene can reduce lipopolysaccharide-induced inflammatory responses by regulating the NF-κB, JAK2/STAT3 and JNK/p38 MAPK signaling pathways in macrophages. In a study of endoplasmic reticulum stress in weaned piglets, it was also found that β-carotene inhibited the activation of the IRE1-JNK/p38 MAPK signaling pathway in a dose-dependent manner, thereby enhancing the body's immune capacity and reducing the inflammatory response [24].
4.4 Immune regulation function
Beta-carotene can be converted into vitamin A in the body, participate in the development of the immune system, and enhance the body's immunity [25-26]. It can also directly exert an immune regulatory function by regulating gene expression and immune responses, promoting the proliferation of B lymphocytes and T lymphocytes, and the production of macrophages and cytokines [27]. Ishida et al. [28] found that adding 500 mg/d β-carotene to the diet of Japanese black cattle can increase the concentration of IgG1 in colostrum and the concentration of β-carotene in plasma. Akkara et al. [29] found that in a model of liver toxicity induced by bromobenzene in male Wistar rats, pretreatment with 10 mg/kg BW of β-carotene by gavage can significantly alleviate liver and kidney damage and the expression of inflammatory factors.
4.5 Other functions
Beta-carotene also promotes pigment deposition, improves meat quality, enhances reproductive performance and affects intestinal microorganisms [30]. Carotenoids such as beta-carotene can promote the deposition of pigments in aquatic animals [31] and egg yolks [32], improving product quality and price. Lopez et al. [16] found that beta-carotene can promote the expression and release of GnRH, stimulate the secretion of reproductive hormones such as luteinizing hormone (LH) and follicle-stimulating hormone (FSH), improve follicle quality, and enhance reproductive capacity. Henriquez et al. [33] found that high-carotene corn can increase intramuscular fat and improve pork quality without changing the lean meat rate of the carcass. Yuan et al. [34] found that supplementing the sow diet with β-carotene can significantly increase the abundance of the phylum Firmicutes in the intestine and increase the number of beneficial bacteria.
5 Research progress of β-carotene in livestock and poultry farming
As research on β-carotene in animal production continues to deepen (Table 1), it has been approved for use as a feed additive in 52 countries and regions around the world.
5.1 Application of β-carotene in pig farming
Beta-carotene can alleviate the stress of piglets being weaned, enhance immunity and promote piglet growth. Wu Min [35] found that beta-carotene may regulate the TLR4/MyD88/NF-kb signaling pathway to reduce lipopolysaccharide-induced inflammatory damage in IPEC-J2 cells. Adding 200 mg/kg β-carotene to the diet of 28-day-old weaned piglets can significantly increase the relative expression of CCL25 and IgASC genes, promote the secretion of antibodies by piglet epithelial cells, reduce the expression of pro-inflammatory cytokines, and improve growth performance. In a study by Hong Pan [36], it was found that β-carotene can significantly reduce MDA levels in the intestines of weaned piglets, enhance GSH-Px and SOD activity, and dose-dependently inhibit the expression levels of proteins such as phosphorylated JNK and p38MRK, indicating that β-carotene can relieve endoplasmic reticulum stress response and apoptosis in intestinal cells of weaned piglets, exert anti-inflammatory effects, and reduce weaning stress damage. The results of a study by Zhang Xiaoyin et al. [37] showed that adding β-carotene to the feed of sows in the late stages of pregnancy can increase the β-carotene content of the feces and also increase the concentration of IgA in the serum and milk, thereby enhancing the immune function of the sow.

Yu et al. [38] found that the use of β-carotene as an antioxidant can improve oocyte quality and even ovarian function, which suggests that β-carotene can improve animal reproductive performance. This hypothesis was verified in a study by Chen et al. [39], in which the addition of β-carotene to the sow's diet significantly increased the concentration of immunoglobulins such as colostrum IgM, IgG and other immunoglobulin concentrations, and improved the birth weight and individual weight of piglets. Kostoglou et al. [40] compared the effects of adding 400 mg/kg β-carotene to the feed of sows at different stages of pregnancy or injecting 200 mg/kg β-carotene into sows three times. They found that the injection group and the β-carotene group both increased the weaning weight of piglets. In Yuan et al. [34] study, adding 30 mg/kg and 90 mg/kg β-carotene to the diet of sows from 90 days of gestation to farrowing did not significantly improve reproductive performance such as litter size and piglet birth weight, but showed the same trend of improvement.
In addition, β-carotene also has a certain effect on pork quality. The results of a study by Yuan Dezhi et al. [41] showed that adding β-carotene and sodium bicarbonate to the diet of fattening pigs can reduce drip loss and extend the shelf life of pork. The mechanism of action may be that the antioxidant effect of β-carotene reduces lipid oxidation in cell membranes, maintaining cell integrity and thereby reducing the exudation of intracellular fluid.
5.2 Application of β-carotene in poultry farming
In poultry farming, β-carotene is widely used to improve the color of poultry meat and egg products and increase the value of poultry products. Li Junying et al. [42] found that adding an appropriate amount of β-carotene to the diet of laying hens can increase egg production and the average egg weight, as well as improve the egg yolk color, with the best effect being achieved when the additive amount is 150 mg/kg. The study found that there was no significant difference in the growth and egg-laying performance of laying hens under free-range and cage-reared conditions, but the proportion of egg yolk in the free-range group was significantly higher than that in the cage-reared group [43], which may be related to the better absorption and utilization of β-carotene under free-range conditions. Khan et al. [44] found that the amount of β-carotene deposited in poultry egg yolks from the feed was very small, generally only 0.16% to 0.66%. Kristina et al. [45] showed that only 8.85% of the β-carotene in the hybrid maize was deposited in the egg yolk.
Beta-carotene can promote the development of young birds' immune organs and growth. Liu Haiyan et al. [46] found that adding beta-carotene to the diet can significantly increase the daily weight gain and tibia length of early-stage Hy-Line brown chicks, and improve the thymus index, spleen index and bursa index. Ji Yubin et al. [47] found that on the 21st and 42nd days of feeding, the β-carotene and carrot residue groups both significantly increased the IgA content in the serum of Hy-Line brown chicks.

Other studies have shown that β-carotene has a promoting effect on the deposition of pigments in the skin and eggs in poultry farming, but the amount deposited itself is not high. It also has the effect of preventing the deterioration of chicken meat and eggs, and can also increase antibodies and improve immunity [48].
5.3 Application of β-carotene in ruminant farming
Beta-carotene is prone to structural breakdown after stimulation by light and heat, and has poor stability, which limits its application in ruminant production [49]. Nevertheless, there is also a large amount of scientific research that shows that beta-carotene still has a positive effect on the production performance of ruminants. Chen Liqing [50] found that adding different levels of β-carotene to the diet can increase milk production and lactation efficiency in dairy cows, and increase the total solids, milk protein and lactose content in milk. β-carotene also has a positive effect on improving the meat quality of ruminants. Jin Qing et al. [51] found that supplementing β-carotene in the diet can improve the slaughter rate and net meat yield of beef cattle. Bi Yulin et al. [52] found that as the level of β-carotene added to the feed increased, the brightness of the beef color linearly decreased, improving the color saturation of the meat. Jin et al. [53] found that β-carotene in the diet of beef cattle can inhibit the deposition of back fat by inhibiting fat synthesis and promoting fat hydrolysis.
Adding β-carotene to the diet can improve the breeding rate and conception rate of ruminants and promote reproductive performance. Li Ziyan et al. [54] found that adding β-carotene to the postpartum diet of dairy cows can shorten the time to the first estrus after calving and the time to insemination, increase the trend of the conception rate during estrus, and reduce the number of inseminations and the incidence of mastitis. The effect of adding 400 mg/head is better than that of adding 200 mg/head. Kawashima et al. [55] found that Dairy cows that orally consumed 2,000 mg of β-carotene per day from 21 days before expected calving to before calving had a significantly higher number of active corpus luteum 21 days after calving than the control group, indicating that β-carotene supply may support the occurrence of corpus luteum activity in early lactation. Li Qiufeng et al. [56] showed that β-carotene can significantly improve the viability of Holstein bull semen under high temperatures. Eriani et al. [57] added different levels of β-carotene to fresh semen from Aceh swamp buffaloes before freezing. They found that after adding 0.625% (w/v) β-carotene, cell viability, acrosome integrity and plasma membrane integrity were the highest. The pregnancy rate of artificial insemination with buffalo semen treated with 0.625% β-carotene was 50%.

6 Summary
β-carotene plays an important biological function in animal nutrition, immunity and body health. In recent years, significant progress has been made in the biosynthesis and natural extraction of various nutrients. With the continuous improvement of the animal breeding industry chain and the gradual expansion of the demand for green breeding, the development of safe and efficient functional feed additives will become the main trend in the technological development of the modern feed industry. The specific biological functions of β-carotene in animals, its impact on intestinal health and the related mechanisms of action have not yet been fully clarified and require further research. However, β-carotene and feed ingredients and premixes rich in β-carotene will have broad application prospects in China's livestock and poultry farming industry.
Reference:
[1]Lobato K B, Paese K, Forgearini J C, et al. Characterisation and stability evaluation of bixin nanocapsules[J]. Food Chem, 2013, 141(4): 3906-3912.
[2]Khoo H R, Prasad K N, Kong K W, et al. Carotenoids and their isomers: Color pigments in fruits and vegetables[J]. Molecules, 2011, 16(12): 1710-1738.
[3] Xiang Xuebing, Wang Ping, Zhai Dewei, et al. Research and analysis of the carotenoid industry [J]. Chemical Industry Management, 2020(14):166-170.
[4] Zuo Shanshan, Li Yang, Ma Lu, et al. Research progress on the biological activity of β-carotene [J]. Journal of Food Safety and Quality, 2020, 11(21):7694-7699.
[5]Papadaki E, Mantzouridou F T. Natural β-carotene production by blakesleatrispora cultivated in spanish-style green olive processing wastewaters[J]. Foods, 2021, 10(2):327.
[6]Ludmila B R, Joanna H. β-Carotene—properties and produ- ction methods[J]. Food Qual Saf, 2018, 2: 69-74.
[7]Paula M B, Marielle M, Charles D, et al. Comparison of the bioavailability and intestinal absorption sites of phytoene, phytofluene, lycopene and β-carotene[J] . Food Chem, 2019, 300: 125232.
[8]Loredana Q, Elena G, Brianna K, et al. Interplay between β-carotene and lipoprotein metabolism at the maternal-fetal barrier[J] . BBA-Mol Cell Biol L, 2019, DOI: 10.1016/j. bbalip.2019.158591.
[9]Von Lintig J, Sies H. Carotenoids[J]. Arch Biochem Biophys, 2013, 539(2): 99-101.
[10]Turner T, Burri B J, Jamil K M, et al. The effects of daily consumption of β-cryptoxanthin-rich tangerines and β-carotene- rich sweet potatoes on vitamin A and carotenoid concentrations in plasma and breast milk of Bangladeshi women with low vitamin A status in a randomized controlled trial[J]. Am J Clin Nutr, 2013, 98(5): 1200-1208.
[11]Moore T. Vitamin A and carotene: the absence of the liver oil vitamin A from carotene. VI. The conversion of carotene to vitamin A in vivo[J]. Biochem J, 1930, 24(3): 692-702.
[12]KimY K, WassefL, Chung S, et al. β-Carotene and its cleavage enzyme β-carotene-15, 15'-oxygenase (CMOI) affect retinoid metabolism in developing tissues[J]. FASEB J, 2011, 25(5):1641- 1652.
[13]Elvira-Torales L, Garcia-Alonso J, Periago-Caston M J, et al. Nutritional of carotenoids and their effect on liver health:A review[J]. Antioxidants, 2019, 8: 229.
[14] Zhang Xianglun, Fan Ximei, You Wei, et al. Effect of dietary β-carotene supplementation on the antioxidant capacity of beef cattle [J]. Shandong Agricultural Science, 2017, 49(6): 119-122.
[25] Zhang Chengyue, Deng Zeyuan, Li Hongyan. Activity of carotenoids in scavenging superoxide anions and hydrogen peroxide free radicals [J]. Journal of Nanchang University (Science Edition), 2018, 42(2): 129-133.
[16]Lopez F N, Meza H C, Perez M C, et al. Precision Beta-carotene supplementation enhanced ovarian function and the LH release pattern in yearling crossbred anestrous goats[J]. Animals, 2020, 10(4):659.
[17]Liang R, Shoemaker C F, Yang X, et al. Stability and bioacc- essibility of β-carotenein nanoemulsions stabilized by modified starches[J]. JAgric Food Chem, 2013, 61(6): 1249-1257.
[18] Zhou Tong, Liu Haijun, Xu Ping, et al. Effects of β-carotene on learning and memory and hippocampal caspase-3 and phosphorylated tau expression in rats with obstructive sleep apnea syndrome [J]. Journal of Clinical Neurology, 2019, 32(1): 50-53.
[19]Zhou L H, Ouyang L, Lin S Z, et al. Protective role of β-carot- ene against oxidative stress and neuroinflammation in a rat model of spinal cord injury[J]. IntImmunopharmacol, 2018, 61: 92-99.
[20]YAmamoto M, Kensler T W, Motohashi H. The KEAP1-NRF2 system: athiol-based sensor-effector apparatus formaitaining redox homeostasis[J]. Physiol Rev, 2018, 98(3): 1169-1203.
[21] Qu Huiming, Wang Ying, Zhao Bo, et al. Protective effect of β-carotene on H2O2-induced liver damage in zebrafish [J]. Food Science, 2019, 40(5): 162-166.
[22] Qiao Dong, Pang Guangchang, Li Yang. The regulatory effect of β-carotene on the immune system [J]. Modern Food, 2016(50): 96-97.
[23]Li R N, Pan H, Xin Z. β-carotene attenuates lipopolysac- charide-induced inflammation via inhibition of the NF-κB, JAK2/STAT3 and JNK/p38 MAPK signaling pathways in macrophages[J]. Anim Sci J, 2018, 90(4): 1-9.
[24]Li R N, Yang Y, Hong P, et al. β-carotene attenuates weaning- induced apoptosis via inhibition of PERK-CHOP and IRE1- JNK/p38 MAPK signalling pathways in piglet jejunum[J] . J Anim PhysiolAnim Nutr, 2020, 104(1): 280-290.
[25]Honda K, Littman D R. The microbiota in adaptie immune homeostasis and disease[J]. Nature, 2016, 535(7610): 75-84.
[26]Xiao S, Li Q, Hu K, et al. Vitamin Aand retinoic acid exhivit protective effects on necrotizing enterocolitis by regulating intestinal flora and enhancing the intestinal epithelial barrier[J]. Arch Med Res, 2018, 49(1): 1-9.
[27]Milani A, Basirnejad M, Shahabazi S, et al. Carotenoids: biochemistry, pharmacology and treatment[J]. Br J Pharmacol, 2017, 174(11): 1290-1324.
[28]Ishida M, Nishijima Y, Ikeda S, et al. Effects of supplemental β-carotene on colostral immunoglobulin and plasma β-carotene and immunoglobulin in Japanese Black cows[J]. Anim Sci J, 2018, 89(8): 1102-1106.
[29]Akkara P J, Prince, Sabina E. Pre-treatment with beta carotene gives protection against nephrotoxicity induced by bromobenzene via modulation of antioxidant system, pro-inflammatory cytokines and pro-apoptotic factors[J]. Appl Biochem Biotechnol, 2020, 190(2): 616-633.
[30] Li Chao, Jia Bingyu, Gao Min, et al. Biological functions and mechanisms of β-carotene [J]. Journal of Animal Nutrition, 2018, 30(8):2931-2937.
[31]Angell A, Denys R, Mangott A, et al. The effects of concen- tration and supplementation time of natural and synthetic sources of astaxanthin on the colouration of the prawn Penaeus monodon[J]. Algal Res, 2018, 35:577-585.
[32] Mao Guoxiang, Li Liang, Su Yanjing, et al. Gong Daoqing. Effect of different pigment additives on egg yolk coloring [J]. China Poultry, 2016, 28(24):140-142.
[33]Henriquez R E, Pena R N,A. SeradjR, et al. Carotenoid intake and SCD genotype exert complementary effects over fat content and fatty acid composition in Duroc pigs[J]. J Anim Sci, 2017, 95(6): 2547-2557.
[34]Yuan X P, Yan J H, Hu Rz, et al. Modulation of gut microbiota and oxidative status by β-carotene in late pregnant sows[J] . Front Nutr, 2020, 7: 612875.
[35] Wu Min. Protective effect and mechanism of β-carotene on lipopolysaccharide-induced inflammation in IPEC-J2 cells [D]. Changchun: Jilin Agricultural University, 2016.
[36] Hong Pan. Effect of β-carotene on endoplasmic reticulum stress and its signaling pathway in the intestine of early-weaned large-breasted piglets [D]. Changchun: Jilin Agricultural University, 2018.
[37] Zhang Xiaoyin, Ji Yubin, Li Yanqiang, et al. Effect of β-carotene on the concentration of immunoglobulin A in the feces, serum and milk of pregnant sows [J]. Journal of Animal Nutrition, 2016, 28(2): 572-578.
[38]Yu S, Zhao Y, Feng Y, et al. β-carotene improves oocyte develo- pment and maturation under oxidative stress in vitro[J]. In Vitro Cell Dev BiolAnim, 2019, 55(7): 548-558.
[39]Chen J, Chen J, Zhang Y, et al. Effects of maternal supple- mentation with fully oxidised β-carotene on the reproductive performance and immune response of sows, as well as the growth performance of nursing piglets[J] . Brit J Nutr, 2021, 125(1):62-70.
[40]Kostoglou P, Kyriakis S C, Papasteriadis A, et al. Effect of β-carotene on health status and performance of sows and their litters[J]. JAnim PhysiolAnim Nutr, 2000, 83(3): 150-157.
[41] Yuan Dezhi, Wang Bin, Liao Bo, et al. Effects of β-carotene and sodium bicarbonate added to the diet of fattening pigs on growth performance and post-slaughter pork quality [J]. Modern Animal Husbandry, 2016(30): 25-27.
[42] Li Junying, Zhan Kai, Wu Junfeng, et al. Effect of β-carotene on the production performance of laying hens [J]. China Animal Husbandry Journal, 2012, 48(11): 49-51.
[43]Skřivan M, Englmaierová M. The deposition of carotenoids and α-tocopherolin hen eggsproduced under a combination of sequential feeding and grazing[J]. Anim Feed Sci Technol, 2014, 190(7): 79-86.
[44]Khan M S, Amin M R, Florian J S. Carotenoid status of poultry egg under different feeding system in bangladesh[J]. Int J Poult Sci, 2017, 16(6): 228-232.
[45]Kristina K, Zlatko J, Darko G. Content and deposition of carotenoids in egg yolk from hens fed diets differentiated in maize hybrid[C] . The XVth European Poultry Conference: Croatian Branch of the World’s Poultry Science Association,
2018: 334.
[46] Liu Haiyan, Ji Yubin, Wang Yulin, et al. Effect of β-carotene supplementation in the diet on the growth performance and immune organs of Hy-Line brown chicks [J]. Heilongjiang Animal Husbandry and Veterinary Medicine, 2016(24): 75-77.
[47] Ji Yubin, Liu Haiyan, Wang Yulin, et al. Effect of β-carotene and radish residue in the diet on immune indicators and blood biochemical indicators of Hy-Line brown chicks [J]. China Feed, 2016(6): 14-17.
[48]SÜLeyman C. The important of beta carotene on poultry nutrition[J]. Selcuk JAgric Food Sci, 2019, 33(3): 256-263.
[49]Yi J, Lam T I, Yokoyama W, et al. Beta-carotene encapsulated in food protein nanoparticles reduces peroxyl radical oxidation in Caco-2 cells[J]. Food Hydrocoll, 2015, 43: 31-40.
[50] Chen Liqing. Study on the effect of adding β-carotene to the diet on the production performance and antioxidant indicators of dairy cows [D]. Hohhot: Inner Mongolia Agricultural University, 2018.
[51] Jin Qing, Bi Yulin, Cheng Haijian, et al. Effect of β-carotene on slaughter performance and carcass quality of beef cattle [J]. Journal of Animal Science and Technology, 2016, 37(5):26-31.
[52] Bi Yulin, Wan Fachun, Jiang Shuzhen, et al. Effects of β-carotene on the production performance, antioxidant function, blood physiological indicators and meat quality of beef cattle [J]. Journal of Animal Nutrition, 2014, 26(5): 1214-1220.
[53]Jin Q, Zhao H B, Liu X, et al. Effect of β-carotene supplem- entation on the expression of lipid metabolism-related genes and the deposition of back fat in beef cattle[J]. Anim Product Sci, 2017, 57(3): 513-519.
[54] Li Ziyan, Tuo Zhengjun, Yang Yudong, et al. Effect of β-carotene on the reproductive performance of dairy cows [J]. Heilongjiang Animal Husbandry and Veterinary Medicine, 2018(4): 69-71.
[55]Kawashima C, Nagashima S, Sawada K, et al. Effect of β-caro- tene supply during close-up dry period on the onset of first postpartum luteal activity in dairy cows[J]. Reproduct Domestic Anim, 2010, 46(6): e282-e287.
[56] Li Qiufeng, Li Jianguo, Wu Yunhai, et al. Effect of dietary zinc, vitamin E and β-carotene supplementation on semen quality and antioxidant indicators in bulls under high temperature conditions [J]. Journal of Animal Science and Veterinary Medicine, 2014, 45(4): 587-595.
[57]Eriani K, Azhar A, Ihdina M, et al. Quality enhancement of Aceh swamp buffalo (Bubalus bubalis) frozen semen by supplementing β-carotene[J] . Trop Anim Sci J, 2018, 41(1): 1-7.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese