What Is Sweetener Luo Han Guo Extract Mogroside?
Luo Han Fruit is a “dual-purpose medicine” that can be used as both medicine and food. It has the function of clearing away heat and moistening the lungs. Mogroside is a natural sweetener in Luohanguo. It is safe and non-toxic[1], has a high sweetness and low calorie content[2,3], and is 200 to 300 times sweeter than sucrose. It can be used as a substitute for sucrose in functional foods[4], especially suitable for the prevention and treatment of diabetes [5]. Mogroside is a tetracyclic triterpene compound with a structure of cucurbitane. Recent studies have shown that not only does mogroside have biological activities such as liver protection [6, 7], immune enhancement [8], anti-inflammation [9, 10], anti-fatigue [11], and antitussive [12], but it also has significant hypoglycemic effects [13, 14].
Mogroside is a kind of sweet glycoside, which is extracted from the fruit of the Luo Han Guo (Siraitia grosvenorii) plant. Luo Han Guo is a vine in the Cucurbitaceae family, and its cultivation conditions are harsh, requiring a warm, humid climate that is not resistant to high temperatures and is afraid of frost [15, 16]. It is mainly distributed in Guangxi, Hunan, Guizhou and other regions of China. The fruit of Luo Han Guo has a low content of sweet glycosides and is difficult to cultivate, so the production cost is high. After being made into a sweet glycoside product, the price is high, making it difficult to be widely used in the food industry. The production of mogroside through biosynthesis technology is an important solution to meet market demand [17, 18]. Biosynthesis technology has made great progress in recent years. Exploring the molecular mechanism of glycoside synthesis will lay the foundation for using synthetic biology to construct cell factories to produce glycosides.

This paper reviews the research on the molecular mechanism of mogroside in regulating blood sugar, and reviews and discusses the synthesis pathway of glycoside molecules and synthetic biology technology.
1 Research on the hypoglycemic effect of mogroside
Mogroside tastes sweet and can regulate sugar metabolism. After being administered to diabetic mice, mogroside can improve their blood glucose levels [19]. Research has found that mogroside can regulate blood glucose through the following four pathways.
1.1 Repair damaged pancreatic β cells
Pyrimidine is an oxygen-containing derivative of pyrimidine that can selectively induce pancreatic β cell damage and apoptosis, thereby inhibiting the synthesis of proinsulin [20]. Zhang Liqin et al. used tetrahydropyrimidine to construct a diabetic mouse model [21]. The drug was administered by gavage, and blood was taken from the orbital region to measure the blood glucose level. Compared with the control group, the Mogroside treatment group had a significant hypoglycemic effect. It is speculated that Mogroside exerts its hypoglycemic effect by repairing pancreatic β cells and increasing insulin secretion.
Intracellularly enriched reactive oxygen species (ROS) can induce islet cell damage. Chen Shanyuan used mouse islet β cells (NIT-1) as the research object [22], cultured NIT-1 cells after administration, and used a flow cytometer to measure the intracellular ROS content. It was found that the ROS level in NIT-1 cells in the administration group was significantly reduced, it is speculated that Mogroside reduces oxidative stress damage by scavenging ROS in pancreatic β cells [23, 24]. Qi Xiangyang et al. used diabetic mice as the drug administration target to study the protective mechanism of Mogroside on mouse pancreatic tissue [25]. After administration, Mogroside was found to reduce the blood glucose concentration of type 1 diabetic mice and improve the degree of pancreatic lesions. the expression levels of IFN-γ and TNF-α in the pancreas decreased, and the number of CD4 lymphocytes in the spleen of mice increased. The experimental results suggest that Luo Han Guo has the potential to repair islet cells.
1.2 Stimulate insulin secretion
The level of insulin secretion is a key factor in maintaining stable blood glucose levels in the body. After a meal, an increase in blood glucose levels will stimulate islet cells to release insulin to regulate blood glucose. He Chaowen et al. used normal mice as the research object to study the fluctuations in blood glucose and insulin secretion after the mice took Mogroside [26]. It was found that there was a correlation between the blood glucose level, insulin secretion and the dose of Mogroside administered. It is speculated that Mogroside exerts its regulatory effect on blood glucose by promoting insulin secretion in the body and lowering blood glucose levels.
Zhou Ying et al. studied the effect of Mogroside V on insulin secretion [27]. The results showed that Mogroside can induce insulin secretion in the insulinoma cell RIN-5F, revealing the effect of Mogroside on insulin secretion at the cellular level and suggesting that Mogroside may have the potential to prevent or treat type 2 diabetes.
1.3 Regulation of adenosine monophosphate-activated protein kinase inhibits the gluconeogenic pathway
Adenosine monophosphate-activated protein kinase (AMPK) is a key protein molecule that regulates the body's energy balance. This protein plays an important role in regulating the body's glucose and lipid metabolism [28]. Studies have found that AMPK is closely related to obesity and the development of type 2 diabetes. AMPK can regulate blood glucose levels by activating the AMPK pathway in the body [29]. After AMPK is activated, it will inhibit the expression of the genes of key enzymes in gluconeogenesis (glucose phosphate isomerase and phosphoenolpyruvate carboxylase), thereby inhibiting the gluconeogenesis pathway [30] and reducing blood glucose levels. Chen Xubing et al. found in an in vitro experiment that Mogroside V cannot directly activate AMPK in HepG2 cells, but when the glycoside is digested in the body and converted to monacolin, monacolin activates AMPK to inhibit the gluconeogenesis blood glucose regulation pathway [31] (Figure 1). This study further clarifies the efficacy and molecular mechanism of Luo Han Guo in lowering blood sugar at the molecular level.
1.4 Inhibits the activity of glycosidase in vivo
Mogroside can regulate blood glucose levels in the body by inhibiting glucosidase activity. There is a large amount of glucosidase distributed on the mucosa of the small intestine. Its function is to degrade polysaccharides such as starch into monosaccharides by hydrolyzing glycosidic bonds. Therefore, when glucosidase activity is reduced, the digestion and absorption of polysaccharide foods by the small intestine is inhibited [32, 33]. Clinically, lowering blood glucose concentration by inhibiting the activity of α-glucosidase on the small intestinal mucosa is an important method for the prevention and treatment of type 2 diabetes [34]. Xia Xing et al. studied the effect of Mogroside on the activity of α-glucosidase [35]. In vitro enzyme kinetic studies showed that Mogroside can inhibit the activity of intestinal α-glucosidase, suggesting that Mogroside can delay the rate of carbohydrate breakdown in the intestine and inhibit glucose absorption by inhibiting the activity of α-glucosidase, thereby avoiding a sharp increase in postprandial blood glucose concentration.
2 Other studies on the activity of mogroside
2.1 Boosts immunity
Cyclophosphamide (CTX) is an alkylating agent immunosuppressant. CTX inhibits the proliferation of both T cells and B cells. Wang Qin [8] and others studied the ability of Mogroside to regulate the immune system of mice. CTX was injected into the abdominal cavity of mice to inhibit the immune system of mice. The dose was 0.75 to 1.5 g/kg/d. Mogroside was given to mice by gavage for 10 days. the proliferation of immune cells and the phagocytic ability of macrophages in mice were measured. It was found that mogrosides can significantly promote the proliferation of CTX immunosuppressed mouse T cells and enhance the phagocytic function of mouse macrophages, promoting the recovery of mouse immune function to normal levels, suggesting that mogrosides may have a certain repair ability for the immune system.
2.2 Anti-fibrosis
Hepatic stellate cells are involved in vitamin A metabolism and are an important site for fat storage in the liver. When the liver is chemically stimulated, mechanically damaged or infected with a virus, hepatic stellate cells will change from a resting state to an activated state. The sustained activation of hepatic stellate cells will induce abnormal cell proliferation, increased secretion of extracellular matrix, and gradual transformation into myofibroblasts. In the process of liver fibrosis, type I collagen can induce the activation and proliferation of hepatic stellate cells, and transforming growth factor β1 (TGF-β1) can promote the transformation of hepatic stellate cells into fibroblasts. Song Kaijuan [36] and others administered LX-2 liver stellate cells with Mogroside at different concentrations and found that the sweet glycoside not only promoted LX-2 cell apoptosis, but also inhibited the secretion of TGF-β1 and type I collagen, thereby inhibiting the development of liver cells towards fibrosis.
2.3 Hepatoprotective effect
Zhu Huiling et al. [37] studied the protective effect of sweeteners on normal human liver cells (LO2). LO2 cells were cultured in a medium containing ethanol for 12 h, and the cell growth state was observed. Cell growth was inhibited in the ethanol group. Pretreatment with sweeteners significantly reduced the toxicity of ethanol to LO2 cells. Cell viability in the intervention group (0–200 μmol/L) increased with increasing concentration of the sweetener. When the LO2 cell membrane is damaged and ruptured, the intracellular enzymes alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) can penetrate into the extracellular medium. Biochemical index testing found that ethanol treatment of LO2 cells can lead to increased ALT and LDH values in the culture medium; In the intervention group, the value of ALT and LDH in the culture medium was significantly reduced by the presence of sweet glycoside, indicating that sweet glycoside can reduce the damage to the cell membrane caused by ethanol and maintain the integrity of the liver cell membrane. Xiao Gang et al. [38] studied the effect of sweet glycoside on the repair of liver damage in mice. An acute liver injury model was constructed using the carbon tetrachloride induction pathway, using lipopolysaccharide and BCG to induce an immune-mediated liver injury model. Serum tests showed that the glycoside reduced the levels of alanine aminotransferase and aspartate aminotransferase in the mice's blood. Pathological examinations found that the glycoside reduced the necrosis and lesions of the liver tissue. The above results suggest that the glycoside may have a protective effect on liver cells and liver tissue.
2.4 Cough suppressant and expectorant
Luohan fruit has a cough suppressant effect, but the components in Luohan fruit that exert this effect are not clear. Wu et al. [39] used Kunming mice to construct an animal model for cough suppression and expectorant, and administered the sweetener to the mice by gavage at a dose of 10 to 30 mg/kg. The effect of the glycoside on the duration of the coughing latency in mice was measured by performing an ammonia-induced cough experiment, and the cough suppression effect of the glycoside was evaluated by counting the number of coughs. An experiment of phenol red excretion in the trachea was also performed to measure the expectorant effect of the drug using the amount of phenol red excreted in the trachea as an indicator. The study found that the glycoside can significantly reduce the number of coughs in mice and increase the amount of phenol red excreted in the trachea, suggesting that the glycoside may have an expectorant effect.
2.5 Anti-allergy
Histamine is formed by the decarboxylation of histidine in the body. As an important small molecule transmitter in the body, it can induce a variety of physiological reactions, including inflammation and allergic reactions. Hossen et al. [40] used histamine and “48/80 compound” to induce an itch response model in ICR mice. After administering the sweet glycoside to mice for four weeks, it was found to significantly reduce the itch response of mice. To further illustrate the mechanism of action of the sweet glycoside, mast cells were cultured after administration, and it was found that a concentration of 0.3 mg/mL of the sweet glycoside could significantly inhibit the release of histamine from mast cells induced by the “48/80 compound”. Given that mogroside itself has antioxidant capacity, it is speculated that mogroside inhibits the release of histamine from mast cells by scavenging superoxide anions, thereby inhibiting allergic reactions.
3 Biosynthesis of Mogroside
Mogroside has potential medicinal value, but the low content of the glycoside component in the fruit, the high production cost, and the high price of the finished product, biosynthetic technology provides a new way of thinking for the production of Mogroside. It is clear that the secondary metabolic pathway of Mogroside is the basis for the in vitro synthesis of Mogroside. In recent years, with the development of molecular biology technology, the key enzymes in the biosynthesis of mogroside, including farnesyl diphosphate synthase, cytochrome P450 monooxygenase, and glycosyltransferase, have been discovered [41], and the activity and function of these enzymes have been characterized [42, 43], providing a theoretical basis for the total synthesis of mogroside and the establishment of a cell factory.
3.1 Structure of Mogroside
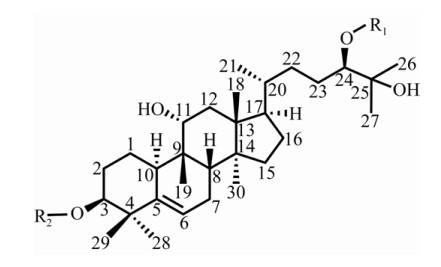
The Mogroside molecule is composed of mogroside alcohol and a glucose moiety (Figure 2) [44]. The number of glucose units linked to the C3 and C24 positions of the mogroside alcohol skeleton is different, which will produce a sweet glycoside molecule with a large difference in taste. 3.2 Synthetic pathway of Mogroside Mogroside is a cucurbitane-type triterpene glycoside, and its synthesis has been initially understood. Its biosynthesis in fruits can be divided into four stages:
3.2.1 Biosynthesis of isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP)
Using acetyl CoA as a raw material, two molecules of acetyl CoA are condensed to form acetoacetyl CoA; under the action of HMG-CoA synthase, acetoacetyl CoA reacts with another molecule of acetyl CoA to to form 3-hydroxy-3-methyl-glutarate monoyl CoA, which is reduced to methylmalonic acid by HMG-CoA reductase [45]. Methylmalonic acid is successively catalyzed by methylmalonyl-CoA kinase, phosphomethylmalonyl-CoA kinase and 5-phosphomethylmalonyl-CoA decarboxylase to successively generate 5-phosphomethyl-D-erythritol, 5-pyrophosphomethyl-D-erythritol and isopentenyl pyrophosphate (IPP) [46], which isomerizes to dimethylallyl pyrophosphate (DMAPP) by isopentenyl pyrophosphate isomerase (Figure 3).
3.2.2 Synthesis of the intermediate product 2,3-oxidosqualene [47]
IPP and DMAPP are catalyzed by geranyl pyrophosphate synthase to form geranyl pyrophosphate (GPP). GPP is catalyzed by farnesyl pyrophosphate synthase (FPS) to synthesize farnesyl pyrophosphate (FPP), which is converted to squalene by squalene synthase (SQS) [48]. which is then converted to 2,3-oxidosqualene by the catalysis of monooxygenase (SE) (Fig. 4).
3.2.3 Synthesis of loganin
Itkin et al. [50] found that 2,3-oxidosqualene can be catalyzed by squalene epoxidase to produce 2,3;22,23-epoxysqualene, which is then cyclized to 24,25-epoxysqualene by squalene epoxidase. and then hydroxy-lated at the C24 and C25 positions by epoxide hydrolase to form a squalene epoxide, which is hydroxylated at the C11 position by cytochrome P450 monooxygenase CYP102801 to form momordin (Fig. 5).
3.2.4 Synthesis of sweet glycosides
The expression level of the UDP-glucosyltransferase (UGT) gene, which is involved in the glycosylation of the aglycone during fruit ripening, is significantly upregulated. and finally the synthesis of the Luo Han Guo triterpene saponin is completed by the addition of sugar groups to the C3 and C24 positions of the aglycone by UDP-G glycosyltransferase [51]. Mogroside has the common aglycone mogroside alcohol, and the difference is mainly the number of glucose units linked at the C3 and the number of glucose units at the C3 and C24 positions. The results of studies by It- kin [50] and Dai Longhai [52] and others show that the UGT74AC1 and UGT720-269-1 glycosyltransferases from Luo Han Guo are responsible for the glycosylation of C3-OH of Luo Han Guo alcohol. In addition, UGT720-269-1 is also involved in the glycosylation of mogroside C24-OH, while UGT94-289-3 is responsible for the extension reaction of the glucose chains at the C3 and C24 positions, and finally synthesizes the sweet glycoside V through a five-step glycosylation process (Figure 6).
4 Mogroside in vivo degradation and metabolism
In order to thoroughly analyze the mechanism of action of the glycosides in the body, in recent years, researchers have carried out a large number of studies on the degradation and metabolism of Mogroside in vivo [53]. Lu Fenglai et al. [54] used human intestinal bacteria to degrade mogroside and found that Mogroside III, under the action of bacteria, successively lost the C3 glucose group and the C24 gentiobioside group, and was converted to Luo Han Guo IIA1 and Luo Han Guo alcohol.

Huang Zhencong et al. [55, 56] placed Mogroside V in artificial gastric juice and intestinal bacterial liquid respectively, and tracked and analyzed its transformation products. found that the glycoside V in artificial gastric juice was hydrolyzed one by one, and was finally converted into the aglycone; under the action of human intestinal flora, the glycoside V would undergo both deglycosylation and glycosylation reactions. The glycoside V was converted into a secondary glycoside through deglycosylation, and the glycoside V was converted into a six-glycoside by glycosylation. In vivo experiments using mice showed that there are significant differences between the metabolites of mogroside V in mouse urine and feces [55]: In mouse urine, mogroside V is excreted in the form of mogroside V, while in mouse feces, mogroside V is converted to hydroxylated and isomerization products. The above research provides an important reference for clarifying the metabolism and transformation pathways of Mogroside in the body.
5 Discussion and outlook
Sweeteners are widely used in the production of food and beverages. Although chemical sweeteners are sugar-free and low in calories, consumers find it difficult to accept them. They believe that sweeteners have a chemically synthesized taste, and on the other hand, they are concerned about their safety and worry that long-term consumption will affect their health. From a safety perspective, the acute toxicity of mogroside is LD50 > 15 g/kg (bw) [57], and it is negative in the Ames mutagenicity test and non-genotoxic [58], making it a safe and non-toxic substance. In the field of food, Mogroside is a natural and good substitute for sucrose. It is highly sweet and low in calories. It is not absorbed by the body after consumption and effectively reduces the intake of energy substances [59], which can meet the needs of diabetic patients and obese people.
In terms of pharmacological research, based on the hypoglycemic activity of the glycosides, scientific researchers have studied the mechanism of glycosides in blood glucose regulation. It is speculated that mogroside regulates blood glucose by stimulating insulin secretion, repairing islet cells, inhibiting gluconeogenesis, and inhibiting the activity of glycosidase. This suggests that glycosides have multiple targets, diverse mechanisms of action, and complex signal pathways involved in the regulation of blood glucose in the body. which pathway plays a leading role in blood glucose regulation, and how to synergistically inhibit blood glucose, related research has not yet been reported and remains to be explored in depth.
Mogroside has potential medicinal value, but it is expensive. Clarifying the biosynthetic mechanism of Mogroside and using cell factories to produce Mogroside is one of the potential ways to mass-produce Mogroside:
In the biosynthesis of mogroside triterpene glycosides, the biosynthetic pathway from acetyl CoA to 2,3-oxidosqualene exists in higher eukaryotes and some microorganisms. Oxidized squalene can be used as a precursor to synthesize biomolecules such as steroids or terpenes. In the Luo Han Guo, 2,3-oxidosqualene is finally converted into the mogroside molecule under the catalytic action of a series of key enzymes. At present, there are many difficulties in the total synthesis of mogroside through the biosynthesis pathway, mainly involving three stages: (1) 2,3-oxidation of squalene to form cucurbitadienol; (2) hydroxylation of cucurbitadienol to form momordinol; (3) glycosylation of momordinol. The relevant enzyme genes have been cloned, expressed and functionally verified, However, there is still a lot of work to be done on how to integrate these foreign genes into microbial cell factories and achieve their efficient and coordinated expression.
The hypoglycemic effect of mogroside provides new ideas for the development of hypoglycemic drugs, and more extensive and in-depth clinical research is needed in the future. The synthesis of mogroside is still in its infancy and faces many challenges. Research and exploration of the anabolic metabolism of Luo Han Guo will lay the foundation for the establishment of a mogroside cell factory.
Reference:
[1]Qin X,et al.Subchronic 90-day oral(gavage) toxicity study of a luo han guo mogroside extract in dogs[J].Food Chem Toxicol,2006,44:2106-2109 .
[2]Murata Y,et al.Sweetness characteristics of the triterpene glycosides in Siraitia grosvenori[J].J Jpn Soc Food Sci, 2006,53 :527-533 .
[3]Jin JS,Lee JH.Phytochemical and pharmacological aspects of siraitia grosvenorii,luo han kuo[J].Orient Pharm Exp Med, 2012,12:233-239 .
[4] Chen DH,et al.Studies and uses of natural non- sugar sweeteners from luo-hanguo (fruit of siraitia grosveno- ri)[J].Nat Prod Res Dev(天然产物研究与开发),1992,4
(1) :72-77 .
[5]Behrens M,et al.Sweet and umami taste :Natural products, their chemosensory targets,and beyond[J].Angew Chem Int Edit,2011,50:2220-2242 .
[6]Xiao G .Experiment study on the hepatoprotective effect of mogrosides[J].Chin J Exp Tradit Med Form,2013,19 :196-200 .
[7]Wang Q,et al.Effects of mogrosides on proliferation of hepatic stellate cell-T6 and hepatofibrosis-related gene [J].Chin Traditi Herbal Drugs ,2013,44 :331- 334.
[8]Wang Q,et al. Regulation on the immunological effect of mogrosides in the mice[J].J Chin Med Mat,2001,24:811-812 .
[9]Di R , et al.Anti-inflammatory activities of mogrosides from momordica grosvenori in murine macrophages and a murine ear edema model[J].J Agr Food Chem,2011,59 :7474 .
[10]Shi D,et al.Protective effects and mechanisms of mogroside V on lps-induced acute lung injury in mice[J].Pharm Biol, 2014,52:729-734 .
[11]Liu DD,et al.Effects of siraitia grosvenorii fruits extracts on physical fatigue in mice[J].Iran J Pharm Res,2013,12 : 115-121.
[12]Chen Y,et al.Functional study of natural food sweet- ener mogrosides[J].China Food Addit, 2006,1 :41-43 .
[13]Suzuki YA,et al.Triterpene glycosides of siraitia grosvenori inhibit rat intestinal maltase and suppress the rise in blood glucose level after a single oral administration of maltose in rats[J].J Agr Food Chem,2005,53 :2941-2946 .
[14]Suzuki YA,et al.Antidiabetic effect of long-term supplemen- tation with siraitia grosvenori on the spontaneously diabetic goto-kakizaki rat[J].Brit J Nutr,2007,97 :770-775 .
[15]Jiang SY,et al.GAP of Siraitia grosvenorii cul- tured in vitro and establishment of its SOP[J].Guihaia,2007,27 :867-872 .
[16]Mo CM,et al.Standard operating procedure on tis- sue-cultured seedlings multiplication of Siraitia grosvenorii (Swingle)C.Jeffrey[J].Lishizhen Med Mater Med Res,2008,19 :2092-2094 .
[17]Reed J,et al.A translational synthetic biology platform for rapid access to gram-scale quantities of novel drug-like mole- cules[J].Metab Eng,2017,42:185-193 .
[18]Pawar RS,et al.Sweeteners from plants—with emphasis on stevia rebaudiana(bertoni) and siraitia grosvenorii(swingle) [J].Anal Bioanal Chem,2013,405 :4397-4407 .
[19]Qi XY,et al.Efficacy study on siraitia grosvenori powder and its extractson reducing blood glucose in diabetic rabbits[J].Food Sci,2003,24:124-127 .
[20]Szkudelski T.The mechanism of alloxan and streptozotocin action in b cells of the rat pancreas[J].Physiol Res,2001, 50:537-546 .
[21]Qi XY,et al.Mogrosides extract from siraitia grosvenori scav- enges free radicals in vitro and lowers oxidative stress,serum glucose,and lipid levels in alloxan-induced diabetic mice [J].Nutr Res,2008,28 :278-284 .
[22]Chen SY,et al.Mechanism of morgroside interve- ning in related oxidative stress damage of pancreatic islet B cell induced by palmitic acid[J].Chin Pharm, 2012,23 :2116-2119 .
[23]Chen SY,et al.Progress in the mechanism of oxi-dative stress damage to islet beta cells and related therapeu- tic drugs in type 2 diabetes[J].Chin Pharm, 2011,22:3533-3536 .
[24]Zhang LQ,et al.Study on in vitro antioxidant ac- tivity of extracts from siraitia grosvenori.Fruits[J].Food Sci,2006,27 :213-216 .
[25]Qi XY,et al.Effect of a siraitia grosvenori extract containing mogrosides on the cellular immune system of type 1 diabetes mellitus mice[J].Mol Nutr Food Res,2006,50:732-738 .
[26]He CW,et al. Rregulation effect of fresh mango- steen saponins on blood sugar[J].Mod Food Sci Tech,2012,28 :382-386 .
[27]Zhou Y,et al.Insulin secretion stimulating effects of mogro- side v and fruit extract of luo han kuo ( siraitia grosvenori swingle) fruit extract[J].Acta pharm Sin,2009,44 :1252- 1257.
[28]Tan MJ,et al.New cucurbitane triterpenoids from bitter mel- on with potent antidiabetic properties associated with activa- tion of ampk[J].Planta Med,2008,74:913-913 .
[29]Zhang LN,et al.Novel small-molecule amp-activated protein kinase allosteric activator with beneficial effects in db / db mice[J].PloS One,2013,8 :e72092 .
[30]Chen XB,et al.Potential ampk activators of cucurbitane trit- erpenoids from siraitia grosvenorii swingle[J].Bioorgan Med Chem,2011,19 :5776-5781 .
[31]Luo Z,et al.In vitro ampk activating effect and in vivo phar- macokinetics of mogroside v,a cucurbitane-type triterpenoid from siraitia grosvenorii fruits[J]. Rsc Adv,2016,6 :7034- 7041.
[32]Ag H.Pharmacology of α-glucosidase inhibition[J].Eur J Clin Invest,1994,24:3-10 .
[33]nal S,et al.Inhibition of α-glucosidase by aqueous extracts of some potent antidiabetic medicinal herbs[J].Prep Bio- chem Biote,2005,35 :29-36 .
[34]Kumar S,et al. Α-glucosidase inhibitors from plants:A natu- ral approach to treat diabetes[J].Pharmacogn Rev,2011,5 : 19-28.
[35]Xia X,et al.Effect of siraitiae fructus extracts from different growth period fruit on postprandial blood glucose in mice[J].Chin J Exp Tradit Med Form,2012,18 :166-170 .
[36]Song KJ,et al.Effect of activation and apoptosis of mogroside on hepatic stellate cell[J].Chin Tradit Pat Med,2014,36 :481-484 .
[37]Zhu HL,et al.Protective effect of mogroside ex- tract on ethanol-induced L-02 hepatocytes damage[J].J Chin Inst Food Sci Technol,2015,15 (1) :13-18.
[38]Xiao G,et al.Protective effect of mogrosides on ex- perimental liver injury in mice[J].Chin Pharm,2018,19 :163-165 .
[39]Wu Y,et al.Study on the relieving cough and elimi- nating phlegm effects of stemoninine combined with mogro- side Ⅴ on mice[J].Chin Pharm ,2017,28 : 1755-1757.
[40]Hossen MA,et al.Effect of Lo Han Kuo(Siraitia grosvenori Swingle) on nasal rubbing and scratching behavior in ICR mice[J].Biol Pharm Bull,2005,28 :238-241 .
[41]Tiwari P,et al.Plant secondary metabolism linked glycosyl- transferases :an update on expanding knowledge and scopes [J].Biotechnol Adv,2016,34:714-739 .
[42]Dai L,et al.Exploiting the aglycon promiscuity of glycosyl- transferase bs-yjic from bacillus subtilis and its application in synthesis of glycosides[J].J Biotechnol,2017,248 :69-76 .
[43] Wang R , et al.Biotransformation of mogrosides[J].Sweeten- ers:Pharm Biotech Appl,2018,153-165.
[44]Li D,et al.Cucurbitane glycosides from unripe fruits of lo han kuo(Siraitia grosvenori) [J].Chem Pharm Bull,2006, 54:1425-1428 .
[45]Netala VR , et al.Triterpenoid saponins :A review on biosyn- thesis,applications and mechanism of their action[J].Int J Pharm Pharm Sci,2015,7 :24-28 .
[46]Zhao CL,et al.Key enzymes of triterpenoid saponin biosyn- thesis and the induction of their activities and gene expres- sions in plants[J].Nat Prod Commun,2010,5 :1147 .
[47]Meng JR,et al.Cloning and sequence analysis of farnesyl pyrophosphate synthase gene in siraitia grosvenorii [J].Chin Tradit Herb Drugs,2011,42 :2512- 2517.
[48]Fett-Neto AG,et al.Biosynthesis of plant triterpenoid sapo- nins:Genes,enzymes and their regulation[J].Mini-Rev Org Chem,2014,11 :292-306 .
[49]Zhang J,et al.Oxidation of cucurbitadienol catalyzed by cyp87d18 in the biosynthesis of mogrosides from siraitia gros- venorii[J].Plant Cell Physiol,2016,57 :1000-1007 .
[50]Itkin M,et al.The biosynthetic pathway of the nonsugar, high-intensity sweetener mogroside v from siraitia grosvenorii [J].P Natl Acad Sci USA,2016,113 :E7619-E7628 .
[51]Yoshikawa S,et al.Transglycosylation of mogroside v,a trit- erpene glycoside in siraitia grosvenori,by cyclodextrin glu- canotransferase and improvement of the qualities of sweetness [J].J Appl Glycosci,2006,52:247-252 .
[52]Dai L,et al.Functional characterization of cucurbitadienol synthase and triterpene glycosyltransferase involved in biosynthesis of mogrosides from siraitia grosvenorii[J].Plant Cell Physiol,2015,56:1172-1182 .
[53]Yang XR , et al.Metabolites of siamenoside i and their distri- butions in rats[J].Molecules,2016,21 :176 .
[54]Yang XW,et al.Biotransformation of mogroside Ⅲ by human intestinal bacteria[J].J Peking Univ :Health Sci ,2007,39 :657-662 .
[55]Lu LF,et al.Stability of mogroside Ⅴ in artificial gastric juice and its metabolism in vitro[J].Guihaia,2015,6:792-795 .
[56]Zhou G,et al.The metabolism of a natural product mogroside v,in healthy and type 2 diabetic rats[J].J Chromatogr B, 2018,1079 :25-33 .
[57]Nong YQ,et al.An overview of research on the ex- traction and pharmacologic action of mogrosides[J].Guangxi J Traditi Chin Med,2008,31 (1) :6-8 .
[58]Su XJ9,et al.Experiments studies on the non-toxic- ity action of mogrosides[J].Food Sci,2005,26(3) :221-224 .
59 [59]Xu Q,et al.Study on normal human body blood sugar and liver enzymes changes affected by oral mogrosides intake [J].Food Sci,2007,28 :315-317 .


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






