What Is Steviol Glycoside?
Sugar is a necessity in daily life, but the current problem of excessive sugar consumption has begun to threaten human health. Excessive consumption of sugar can increase the risk of obesity, high blood pressure, and diabetes [1]. This has led many countries to launch sugar reduction initiatives, such as the sugar tax on sugary drinks in the United States, the United Kingdom and France, and the “fat tax” on products such as chocolate and ice cream in Denmark. These sugar reduction policies have achieved positive results [2].
China's Healthy China Action has also set out clear requirements for reducing sugar. It mentions the need to develop guidelines as soon as possible on the intake of added sucrose in children's food, advocate reducing sucrose intake among the population, encourage the use of natural sweeteners and sweeteners as substitutes for sucrose in the production of food and beverages, and reduce the risk of overweight, obesity and some cardiovascular and cerebrovascular diseases as much as possible. Saccharin was the first sweetener to be used as a sugar substitute in the food industry, but it was gradually phased out because it was harmful to the human body. It was replaced by artificial sweeteners, represented by aspartame.
These artificial sweeteners are difficult to be converted in the human body, so they can be considered as sugar with no calories. They are still widely used in the food industry. However, with continuous research, a type of green and healthy natural sweetener represented by stevioside has attracted people's attention. Stevioside has only 1/3 of the calories of sucrose, but is hundreds of times sweeter, making it an excellent choice for many people with diabetes and high blood pressure. Steviol glycosides are currently used in the production of baked goods, dairy products, beverages and other products. Among the new beverages launched globally in 2019, the amount of the new sweetener steviol glycosides used is second only to sucralose and acesulfame. This shows that steviol glycosides have good prospects for development.

Stevia is a sweetener naturally found in stevia, namely the steviol dihydroacetate glycoside, commonly known as stevioside [3]. The earliest use of this sweetener originated with the indigenous people of Paraguay in South America. Stevioside was successfully isolated from stevia by two French chemists in the 1930s . In the 1970s, Japan began to import the chrysanthemum plant Stevia rebaudiana from South America and successfully cultivate it, and later stevia began to be used as a sweetener in the Japanese food industry.
Soon after, China introduced stevia from Japan and successfully grew it [4]. The sweetening components in stevia are collectively referred to as steviol glycosides [5]. There are many types of steviol glycosides, including mainly rebaudioside A, rebaudioside C and Rebaudioside F, etc. In addition, steviol glycosides such as stevioside A also belong to the stevioside class of compounds [6]. In 2008, high-purity stevioside and rebaudioside A passed the US GRAS safety certification; in 2011, stevioside was adopted by the Committee on Import and Export (CODEX), making it possible to use it as a food additive and publish food use standards; in addition, stevioside steviol glycosides have also passed the EU's safety review and can now be used as sweeteners in the EU. Subsequently, countries such as China, Singapore, and Malaysia have also begun to include steviol glycosides in the list of sweeteners that can be used. In 2013, rebaudioside A, as the most common steviol glycoside compound began to become the world's mainstream and began to be used in major countries around the world. Since 2018, rebaudioside M, known as the “next-generation stevia,” has slowly begun to replace rebaudioside A as the world's mainstream due to its taste being closest to sucrose.
Stevia sugar glycoside compounds all have the same basic framework in their chemical structure – the ent-kaurenoic acid structure. The sweet-tasting steviol glycosides are all diterpenoid glycosides with an ent-kaurane skeleton. In addition, the sweetness of steviol glycosides is also related to their chemical structure. Yang Quanhua [7 ] According to the range of sweetness values of each stevioside compound, the order of sweetness is: type II skeleton > type III skeleton > type I skeleton > type V skeleton > type IV skeleton (Figure 1). The two most common compounds in stevioside are rebaudioside A and stevioside (St). Using sucrose sugar as the standard, the relative sweetness of steviol glycosides is 150 to 300 times, and the relative sweetness of rebaudioside A is at the top of all steviol glycosides, about 250 to 450 times. In addition , low calorie is also a significant characteristic of stevioside. It can be seen that stevioside is a natural and excellent substitute for traditional sweeteners such as sucrose.
1 Stevioside and its derivatives (steviol and its derivatives preparation technology and structural analysis)
Stevioside There are many types of steviol glycoside compounds, and more than 30 have been discovered [6]. The main ones include the more common stevioside and rebaudioside A, as well as rebaudioside M and rebaudioside D, which are gradually becoming the mainstream in the world, and the less abundant rebaudioside R and rebaudioside S. and rebaudioside D, as well as the lower-content rebaudioside R and rebaudioside S. The basic framework of these steviol glycosides is roughly the same, differing only in the chemical structure of the substituents. In addition Steviol and its derivatives steviol and steviol are also being researched. Steviol glycosides and their derivatives not only exhibit excellent properties for use in the food industry, but also have special physiological functions.
1. 1. Extraction methods for stevioside
The extraction techniques for stevioside compounds that have been discovered so far mainly include hot water extraction, ultrasound-assisted extraction, enzymatic methods, and macroporous resin adsorption methods. Among these methods, macroporous resin adsorption is the most widely used. With with the continuous progress of extraction technology, in recent years, there have also been methods such as rapid solid-liquid dynamic extraction [8], two-phase system extraction [9] and supercritical extraction [10] for the extraction of stevioside. Although there are currently many methods for extracting stevioside, but these extraction methods are not applicable to all stevioside types, and each method is only applicable to a specific range.
Because stevioside and rebaudioside A are the two most common compounds in the stevioside glycoside family, most stevioside extraction methods mainly target these two steviosides.
1. 2 Stevioside and its derivatives
Steviol glycosides mainly include stevioside and its derivatives, which are mainly divided into those extracted from nature and those obtained through the biotransformation of existing steviol glycosides and genetic engineering. Steviol glycosides extracted from nature mainly include rebaudioside A, rebaudioside B, rebaudioside C and rebaudioside D, etc. ; the main compounds obtained through the biotransformation of existing stevioside are steviol and isosteviol, which are derivatives of stevioside; and the stevioside compounds obtained using genetic engineering techniques mainly refer to rebaudioside M.
As can be seen from Table 2, the chemical structures of steviosides and their derivatives differ in the substituents at the R1 and R2 positions of their basic skeletons. The stevioside compound with the highest sweetness is rebaudioside A, which is 250 to 450 times as sweet as sucrose. Rebaudioside A has a similar structure to stevioside, with the difference being an extra glucose unit [20]. Rebaudioside D is similar in structure to rebaudioside A, and a series of in vitro experiments can also prove the similarity between the two. Experiments have also shown that rebaudioside D is safer and can be used in food [21]. The molecular weights of the derivatives steviol and isosteviol are lower than those of the listed stevioside glycosides. They are mainly derived from the hydrolysis of stevioside glycosides under alkaline or acidic conditions, and hydrolysis to form ent-kaurane diterpene steviol or ent-beyerane diterpene isosteviol, respectively [22].
Table 2 Summary of stevioside and its derivatives
Types of Stevia and Its Derivatives | Molecular Formula | R1 | R2 | Relative Sweetness |
Stevioside | C38 H60 O18 | β-Glc | β-Glc-β-Glc(2→ 1) | 250 ~ 300 |
Steviol Glycoside | C32 H50 O13 | H | β-Glc-β-Glc(2→ 1) | 100 ~ 125 |
Rebaudioside A | C44 H70 O23 | β-Glc | β-Glc-β-Glc(2→ 1) |β-Glc-β-Rha(2→ 1) | β-Glc-(3→ 1)|β-Glc-β-Glc(2→ 1) -β-Glc(6→ 1) | β-Glc-(3→ 1)| β-Glc-(3→ 1)|β-Glc-β-Rha(2→ 1) -β-Glc(3→ 1) | β-Glc-(3→ 1)|β-Glc-α-Rha(2→ 1) | β-Glc-(3→ 1)| β-Glc-(3→ 1)|β-Glc-β-Xyl(2→ 1) | β-Glc-(3→ 1)|β-Glc-β-Rha(2→ 1) | β-Glc-(3→ 1)| β-Glc-(3→ 1)| β-Glc-(3→ 1) | 250 ~ 450 |
Rebaudioside B | C38 H60 O18 | H | β-Glc-β-Glc(2→ 1) | β-Glc-(3→ 1) | 300 ~ 350 |
Rebaudioside C | C44 H70 O22 | β-Glc | β-Glc-β-Rha(2→ 1) | β-Glc-(3→ 1) | 50 ~ 120 |
Rebaudioside D | C50 H80 O28 | β-Glc-β-Glc(2→ 1) | β-Glc-β-Glc(2→ 1) | β-Glc-(3→ 1) | 200 ~ 300 |
Rebaudioside E | C44 H70 O23 | β-Glc-β-Glc(2→ 1) | β-Glc-β-Glc(2→ 1) | 150 ~ 300 |
Rebaudioside F | C43 H68 O22 | β-Glc | β-Glc-β-Xyl(2→ 1) | β-Glc-(3→ 1) | 250 ~ 300 |
Rebaudioside M | C56 H90 O33 | β-Glc-β-Glc(2→ 1) | β-Glc-(3→ 1) | β-Glc-β-Glc(2→ 1) | β-Glc-(3→ 1) | 200 ~ 350 |
Rebaudioside N | C56 H90 O32 | β-Glc-α-Rha(2→ 1) | β-Glc-(3→ 1) | β-Glc-β-Glc(2→ 1) | β-Glc-(3→ 1) | - |
Rebaudioside R | C43 H68 O22 | - | - | - |
Rebaudioside S | C44 H70 O22 | - | - | - |
Rebaudioside G | C38 H60 O18 | β-Glc | β-Glc-β-Glc(3→ 1) | - |
Rebaudioside H | C50 H80 O27 | β-Glc | β-Glc-β-Rha(2→ 1) -β-Glc(3→ 1) | β-Glc-(3→ 1) | - |
Rebaudioside I | C50 H80 O28 | β-Glc-β-Glc(3→ 1) | β-Glc-β-Glc(2→ 1) | β-Glc-(3→ 1) | - |
Rebaudioside J | C50 H80 O27 | β-Glc-β-Rha(2→ 1) | β-Glc-β-Glc(2→ 1) | β-Glc-(3→ 1) | - |
Rebaudioside K | C50 H80 O27 | β-Glc-β-Glc(2→ 1) | β-Glc-β-Rha(2→ 1) | β-Glc-(3→ 1) | - |
Rebaudioside L | C50 H80 O28 | β-Glc | β-Glc-β-Glc(2→ 1) -β-Glc(6→ 1) | β-Glc-(3→ 1) | - |
Duoxiangmoside A | C38 H60 O17 | β-Glc | β-Glc-β-Rha(2→ 1) | 50 ~ 120 |
Duoxiangmoside B | C38 H60 O17 | H | β-Glc-β-Rha(2→ 1) | β-Glc-(3→ 1) | 40 ~ 60 |
Steviol | C20 H30 O3 | - | - | - |
Isosteviol | C20 H30 O3 | - | - | - |
Note :Glc ,glucose;Rha ,rhamnose;Xyl ,xylose
1. 2. 1 Rebaudioside A
Rebaudioside A (rebaudioside A), with the chemical formula C44 H70 O23, has a basic skeleton with substituents at the R1 and R2 positions, which are β-Glc and β-Glc-[β-Glc (3-1)]-β-Glc ( 2 - 1 ) , with a molecular weight of 967.88 g/mol. Rebaudioside A is the main component of stevioside and is also the most stable component of stevioside [23]; currently, the main methods for the purification and refinement of rebaudioside A include: high-performance liquid chromatography, thin-layer chromatography, membrane separation, capillary electrophoresis, purification and refining processes mainly include: high-performance liquid chromatography, thin-layer chromatography, membrane separation, capillary electrophoresis, droplet countercurrent distribution chromatography, supercritical extraction, and recrystallization [24]. Rebaudioside A shows a more intense sweetness and a more pleasant taste than other stevia glycosides. Europe, the United States, China, South Korea, Brazil and other places have approved the use of rebaudioside A as sweetener. However, because of the problem of poor taste compared with some sweeteners already in use, it has not gained much popularity in China.
Rebaudioside A has special physiological functions. Some studies have shown that rebaudioside A has a strong hypoglycemic effect [25], it may have therapeutic effects on type 2 diabetes, and has a direct effect on pancreatic β cells to produce insulin [20]. In a study of the hemodynamic effects of rebaudioside A, it was found that in healthy individuals with normal blood pressure or low normal mean arterial pressure, no significant changes in mean arterial pressure or heart rate was not observed [26], indicating that laeviglucosan A also has a certain anti-hypertension effect and has a small effect on the body's blood pressure level. Laeviglucosan A can also enhance insulin production, thereby regulating blood sugar and having a healthy glucose-regulating activity [3]. Rebaudioside A also has a strong inhibitory effect on the TPA-induced inflammatory response in mice, indicating that it has certain anti-cancer effects [27 - 28]. In addition, the results of a study by SARAVANAN et al. [29] show that anti-lipid peroxidation, anti-hyperlipidemia and anti-oxidation are also some of the important properties of the natural low-calorie sweetener rebaudioside A.
1. 2. 2 Stevioside
Stevioside, with the molecular formula C38 H60 O18, has substituents at the R1 and R2 positions of the basic skeleton, which are β-Glc and β-Glc- β-Glc(2→ 1), respectively, and a relative sweetness of 250 to 300 times. Stevioside is one of the main ent-kaurene-type diterpene glycosides in the stevia plant. It has been commercially used to sweeten many foods in South America, Japan, China and other places, but the drawback is that it has a certain bitter aftertaste. Currently, stevioside is extracted using essentially the same methods as rebaudioside A, including microwave-assisted extraction, supercritical fluid extraction and pressurized hot water extraction.
Stevioside has a certain anti-inflammatory effect and is a gastroprotective agent. It can block calcium channels to inhibit smooth muscle contraction, reduce stomach abnormalities caused by histamine in rainbow trout, and is a potential cause of reduced acid secretion caused by histamine and inhibition of the action of pepsin [30]. Stevioside has diuretic properties and causes vasodilation, which reduces plasma volume. Some human studies have also shown that stevioside affects the cardiovascular system, causing hypotension and shortening the contraction time, thereby reducing the incidence of stroke. Clinical trials have shown that stevioside can lower systolic and diastolic blood pressure [3]. Some in vivo and in vitro studies have also shown that stevioside has a hypoglycemic effect, which is mainly due to increased insulin secretion [31]. In addition, like rebaudioside A, stevioside also has anticancer activity [32].
1. 2. 3 Rebaudioside D
Rebaudioside D (rebaudioside D), molecular formula C50 H80 O28, has substituents at the R1 and R2 positions of the basic skeleton, which are β-Glc-β-Glc(2 → 1) and β-Glc -[ β-Glc-(3 → 1 )]-β-Glc (2 → 1 ), with a relative sweetness ranking of 200 to 300 times that of sucrose. It is a stevioside compound with a relatively low content in the stevia plant. The metabolism and toxicity of rebaudioside D are similar to those of rebaudioside A, but the metabolic pathway of rebaudioside D is longer and intestinal absorption is low. In in vitro experiments, rebaudioside A and rebaudioside D solutions exhibit similar stability in simulated gastric juice and intestinal juice, and are easily hydrolyzed by intestinal bacteria collected from the cecum [ Rebaudioside A and Rebaudioside D solutions exhibit similar stability in simulated gastric and intestinal fluids, and are easily hydrolyzed by intestinal bacteria collected from the cecum [21].
1. 2. 4 Rebaudioside M
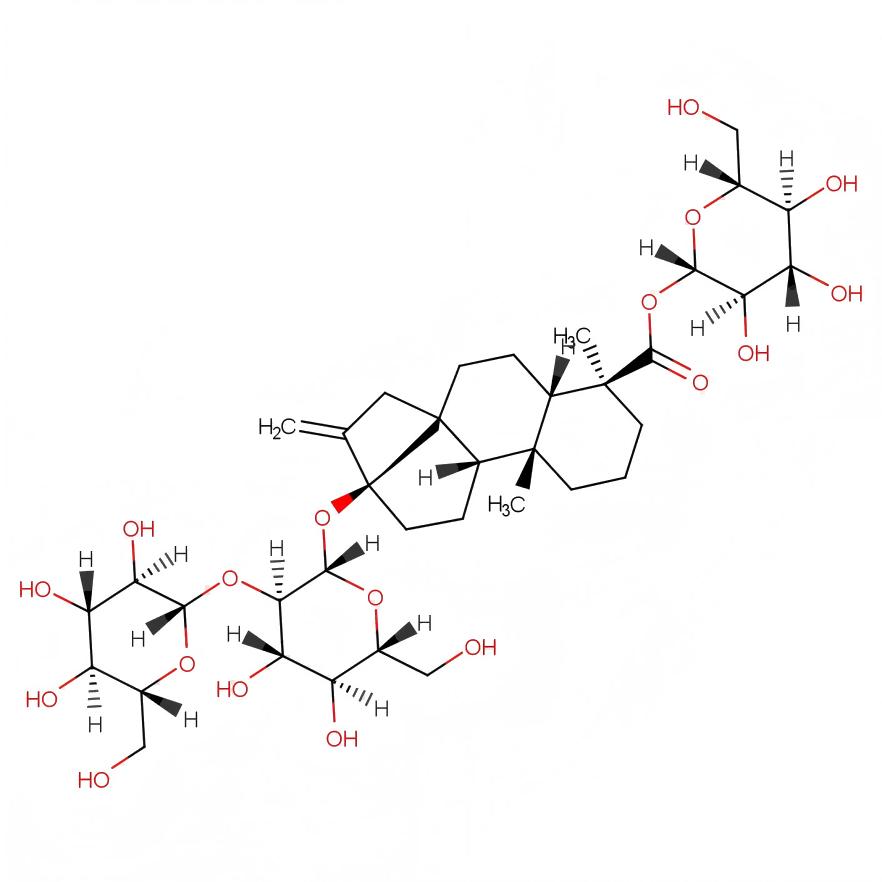
Rebaudioside M (rebaudioside M), with the molecular formula C56 H90 O33, β-Glc-[ β-Glc-(3 → 1 )]-β-Glc (2 → 1 ) is its basic framework. The substituents at the R1 and R2 positions have a relative sweetness of 200 to 350 times. The structure of rebaudioside M is shown in Figure 5. In 2014, PRAKASH et al. reported a new stevioside glycoside compound, rebaudioside M [33]. Rebaudioside M has a clean sweetness, with a slightly bitter or licorice-like aftertaste, and is closest to sucrose in terms of mouthfeel. Rebaudioside M is most stable in a pH 4 to 8 solution, significantly unstable at pH < 2, and its stability decreases with increasing temperature. Its stability is very similar to that of rebaudioside A [34]. In addition, Rebaudioside M is considered safe by the US Food and Drug Administration.
Rebaudioside M is found in very small quantities in nature. There are three main ways to obtain it. The first way is to use genetically modified yeast to obtain it from stevia extract, which has the advantage of high purity. The second way is to obtain it from glucose through fermentation by genetically engineered yeast, but the final rebaudioside M obtained is no longer of natural origin. is no longer of natural origin. The third method is to breed stevia varieties with higher levels of rebaudioside M through continuous improvement, so that large quantities of rebaudioside M can be extracted.
1. 2. 5 Steviol and isosteviol
Steviol (C20H30O3) and isosteviol (C20H30O3), both with a molecular weight of 318.2 g/mol, are derivatives of the stevioside compound. Their structures are shown in Figure 6.
Steviol has anti-inflammatory and hypoglycemic physiological functions. It can directly stimulate the secretion of insulin by pancreatic β cells and INS-1 cells [34–35]. Steviol also has anti-infective effects on colonic epithelial cells. Isosteviol can inhibit angiotensin II-induced cell proliferation and endothelin I secretion. It can also reduce the production of reactive oxygen species and has a certain antioxidant effect [23].
2. Application of stevioside in the food industry
Sucrose is the most common sweetener in the food industry, but its extensive use can lead to an increase in postprandial blood glucose, which can cause obesity and increase the risk of cardiovascular disease. In view of these problems, people have begun to seek new sweeteners to replace traditional sweeteners in the food industry. Stevia, known as the “third-generation healthy sugar source for humans”, is a purely natural, low-calorie, high-intensity sweetener with high safety. It has been found to be an effective substitute for traditional sweeteners and is used as a healthy sweetener in the food industry. Currently, steviol glycosides have been used in baking, beverages, dairy products, candy and other products.
2. 1 Stevioside in baked goods
Baked goods mainly refer to cakes, bread, snacks, etc. Sugar is an indispensable ingredient in the preparation of baked goods. The most common one is sucrose, which can improve the texture and taste of the product. However, long-term consumption of large amounts of sucrose can significantly increase the risk of obesity, tooth decay, and cardiovascular disease. Steviol glycosides, as a new type of natural sweetener, can effectively improve this situation due to their low calorie and high sweetness. In addition, steviol glycosides have high thermal stability, can maintain their stability throughout the baking process, and can be heated to 200 °C. They do not ferment or undergo browning reactions during cooking, can well maintain the flavor of the product, reduce calories, and make it possible to extend the shelf life, broadening the application fields of baking [36]. KARP et al. [37] replaced 20% of the sucrose in chocolate muffins with stevioside, and the cocoa flavor and sweet taste of the muffins were improved.

2. 2 Stevioside in beverages
Juice drinks, carbonated drinks and other beverage products contain a lot of sugar, and long-term consumption can lead to an increase in obesity. Considering these adverse effects, many beverage companies have begun to add steviol glycosides as sweeteners during the beverage production process. For example, Rebaudioside A has been used in the production of beverages by Coca-Cola, the world's largest distributor of fruit drinks [19]. Coca-Cola has successfully reduced the calories in Coca-Cola Life by using stevia as a sweetener; Nestlé has also started to add stevia to its fruit drink Sanpellegrino to replace 40% of the sugar; PepsiCo has also launched a product called 7UP with stevia added.
In addition, some of the more common drinks on the market, such as Xiaomingtong and Nongfu Spring tea, have also begun to use steviol glycosides to replace some of the sugar and thus reduce the sweetness of the product. Although many beverage companies have begun to develop new products using steviol glycosides, there is a risk that consumers will lose interest due to the new formula. Currently, the most widely used sweeteners in the beverage market are steviol glycosides and rebaudioside A. Their addition can achieve the effect of low calories while also sweetening the drink. Most importantly, they can effectively reduce the risk of adverse phenomena such as obesity. For example, peach juice is prepared by mixing stevia (160 mg/L) and sucrose (56 g/L). Compared with a control sample containing 9% sucrose, the calorie content can be reduced by 25% without affecting the sensory quality of the product [30].

2. 3 Stevioside in dairy products
Dairy products mainly include liquid milk, ice cream, cheese and other dairy products. Stevioside is a suitable choice for dairy products because it can maintain its stability after heat treatment [30]. Among dairy products, ice cream is one of the most popular frozen dairy products. During the production of ice cream, its texture, viscosity, and taste are all affected by the sweetener. The most commonly used sweetener in ice cream production is sucrose, but due to the health effects of sucrose, people have begun to use steviol glycosides in ice cream production. Studies have shown that ice cream produced using a mixture of stevia glycosides and sucrose has a better sensory score than ice cream produced using only stevia glycosides [38 - 39]; in addition, the use of stevia glycosides in combination with sucrose has also been found to give a better mouthfeel in some yogurt products. In Xu Zeqi's study [40] on low-sugar fermented soy yogurt with stevioside, the yogurt fermented for 4 hours with 30% sucrose replaced with stevioside not only had a better taste, smell and color, but also had higher nutritional value and was suitable for consumption by diabetic patients and people with dental caries.

2. 4 Stevioside modification research
Although stevioside is a sweetener of natural origin with the advantages of being low in calories and high in sweetness and stable in nature, its sweetness still differs from that of white sugar or sugar alcohols. It has a slight bitter taste and a poor aftertaste, especially when used in hot-brewed products, where the aftertaste becomes more pronounced. Improving the taste quality of stevioside through modification is currently the common goal of sweetener companies. The main modification methods are chemical modification, enzymatic modification and microbial transformation.
2. 4. 1 Chemical modification
Chemical modification refers to changing the molecular structure of steviol glycosides through chemical reactions, thereby changing their physical and chemical properties and organoleptic qualities. It mainly involves improving the taste quality of stevia by changing the sugar groups attached to the base. Chemical modification is rarely studied at present due to the harsh reaction conditions, the many synthetic steps, and safety reasons.
2. 4. 2 Enzymatic modification
Enzyme modification refers to the introduction of a glucose group into stevioside through the transglycosylation or hydrolysis of an enzyme in order to improve its taste. The enzymes used include cyclodextrin glucanotransferase, β-galactosidase, dextrin glucanohydrolase, etc. The product prepared using this method is glucose-based stevioside, which is a food flavoring. Although the taste of steviol glycosides is improved after being modified by the above-mentioned enzymatic method, the relative sweetness is also significantly reduced. Generally, the relative sweetness of the modified product is about 50-150 times that of white sugar.
2. 4. 3 Microbial transformation method
The microbial transformation method refers to the use of microbial metabolic processes to transform stevioside. Most current research uses enzymes in microorganisms to modify stevioside. DE, etc. [41] selected a strain of the fungus Gibberella fujikuroi and used a culture medium with stevioside Stv as the sole carbon source in the culture medium to induce the fungus to produce enzymes, and achieved the partial conversion of stevioside Stv into RA, which has a better taste. Solid fermentation of Aspergillus aculeatus with bran culture medium produced an enzyme solution that convert the Stv and rebaudioside C in stevioside into steviol (SV) in the form of precipitation within 10 h. In this way, the system is effectively enriched with the better-tasting RA [42].
3 Outlook
China is the world's second largest sugar producer, but in recent years, health problems caused by sugar consumption have gradually attracted people's attention, and “sugar-free” and “low-calorie” have become the pursuit of health. In order to meet consumer demand, new sweeteners must be continuously developed to improve the situation. Steviol glycosides, as a new type of green and healthy sweetener, have the advantages of being low in calories and high in sweetness. They not only solve the shortcomings of traditional sweeteners, but also meet consumers' requirements for taste. They can effectively replace traditional sweeteners, meet people's dietary requirements for health, and have great development prospects.
Reference:
[1]LU W Y ,WANG J ,MENG Q J ,et al. Analysis of current situation and development trend of sugar reducing products [ J] . Sugarcane and Canesugar ,2020 ,49 (4 ) :83 - 91
[2]MI Y Q ,WU J ,LIANG X F. Enlightenment from the sugar reduction policy at abroad on control and prevention of chronic diseases in Chi- na[J] . Chinese Health Service Management ,2017 ,34 (4 ) :247 - 248 ;251.
[3]MATHUR S , BULCHANDAN N , PARIHAR S , et al. Critical Review on Steviol Glycosides:Pharmacological ,Toxicological and Ther- apeutic Aspects of High Potency Zero Caloric Sweetener[J] . Interna- tional Journal of Pharmacology,2017 ,13 (7 ) :916 - 928.
[4] FENG X. Study on extraction of stevio glycosides and purification of rebaudioside A[ D] . Nanchang:Nanchang University ,2012.
[5]CHEN J M. In vitro metabolism and biological activity of steviol gly- cosides[ D] . Wuxi :Jiangnan University ,2019.
[6] WÖLWER-R U. The leaves of Stevia rebaudiana ( Bertoni ) , their constituents and the analyses thereof:a review. [J] . Journal of Agri- cultural and Food Chemistry,2012 ,60 (4 ) :886 - 895.
[7]YANG Q H. Analysis of the chemical constituents and its properties of Stevia rebaudiana [ D] . Beijing : Beijing University of Chemical Technology ,2012
[8] GALLO M ,VITULANO M ,ANDOLFI A ,et al. Rapid Solid-Liquid Dynamic Extraction ( RSLDE ) : a New Rapid and Greener Method for Extracting Two Steviol Glycosides ( Stevioside and Rebaudioside A) from Stevia Leaves[J] . Plant Foods for Human Nutrition ,2017 , 72 (2 ) :141 - 148.
[9] ABOLGHASEMBEYK T , SHAHRIARI S , SALEHIFAR M. Extraction of stevioside using aqueous two-phase systems formed by choline chloride and K3 PO4 [ J] . Food and Bioproducts Processing ,2017 , 102 :107 - 115.
[10]AMEERK , CHUN B S , KWON J H. Optimization of supercritical fluid extraction of steviol glycosides and total phenolic content from Stevia rebaudiana ( Bertoni) leaves using response surface method- ology and artificial neural network modeling [ J] . Industrial Crops and Products ,2017 ,109 :672 - 685.
[11]MARTÍNEZ-ALVARADO J C , TORRESTIANA-SÁNCHEZ B , AGUILAR-USCANGA M G. Isolation of steviol glycosides by a two- step membrane process operating under sustainable flux [ J] . Food and Bioproducts Processing ,2017 ,101 :223 - 230.
[12]BURSAC' KOVAEVIC' D ,BARBA F J ,GRANATO D ,et al. Ga- lanakis ,Zoran Herceg , Verica Dragovi-Uzelac , Predrag Putnik. Pressurized hot water extraction (PHWE) for the green recovery of bioactive compounds and steviol glycosides from Stevia rebaudiana Bertoni leaves[J] . Food Chemistry,2018 ,254 :150 - 157.
[13]BURSAC' KOVAEVIC' D ,MARAS M ,BARBA F J ,et al. Innova- tive technologies for the recovery of phytochemicals from Stevia re- baudiana Bertoni leaves:A review [J] . Food Chemistry ,2018 ,268 : 513 - 521.
[14]MILANI G , VIAN M , CAVALLUZZI M M , et al. Ultrasound and deep eutectic solvents:An efficient combination to tune the mecha- nism of steviol glycosides extraction. [J] . Ultrasonics Sonochemis- try,2020 ,69 :105225.
[15] LIU Y F ,DI D L ,BAI Q Q ,et al. Preparative separation and purifi- cation of rebaudioside a from steviol glycosides using mixed-mode macroporous adsorption resins. [ J] . Journal of Agricultural and Food Chemistry ,2011 ,59(17) :9 629 - 9 636.
[16] HUANG X Y ,FU J F ,DI D L. Preparative isolation and purifica- tion of steviol glycosides from Stevia rebaudiana Bertoni using high- speed counter-current chromatography[J] . Separation and Purifica- tion Technology,2010 ,71(2) :220 - 224.
[17]LIU Y ,WANG G ,HUANG X ,et al. Use of O-carboxymethyl chi- tosan in high-speed counter-current chromatography :a novel additive for a biphasic solvent system [ J] . New Journal of Chemistry , 2014 ,38(3) :1 150 - 1 157.
[18]XU S H ,WANG G Y ,GUO R L ,et al. Extraction of steviol glyco- sides from Stevia rebaudiana ( Bertoni) leaves by high-speed shear homogenization extraction[J] . Journal of Food Processing and Pres- ervation ,2019 ,43(12) :e14250.
[19] CASTRO-MUOZ R ,DÍAZ-MONTES E ,CASSANO A ,et al. Mem- brane separation processes for the extraction and purification of steviol glycosides :An overview [ J] . Critical Reviews in Food Sci- cence and Nutrition ,2021 ,61(13) :2 152 - 2 174.
[20] GUPTA E ,PURWAR S ,SUNDARAM S ,et al. Stevioside and re- baudioside A-predominant ent-kaurene diterpene glycosides of ther- apeutic potential :A review [ J] . Czech Journal of Food Sciences , 2017 ,34(4) :281 - 299.
[21]NIKIFOROV A I ,RIHNER M O ,EAPEN A K ,et al. Metabolism and toxicity studies supporting the safety of rebaudioside D[J] . In- ternational Journal of Toxicology,2013 ,32(4) :261 - 273.
[22]WANG M W ,LI H ,XU F X ,et al. Diterpenoid lead stevioside and its hydrolysis products steviol and isosteviol :Biological activity and structural modification[J] . European Journal of Medicinal Chemis- try,2018 ,156 :885 - 906.
[23] SINGH D P , KUMARI M , PRAKASH H G , et al. Phytochemical and pharmacological importance of stevia : A calorie-free natural sweetener[J] . Sugar Tech ,2019 ,21(2) :227 - 234.
[24] SUN C F ,LI J W. Research progress of stevioside [ J] . Food sci- ence ,2010 ,31(9) :338 - 340
[25]SARAVANAN R ,VENGATASH B K. V , RAMACHANDRAN V. Effect of Rebaudioside A ,a diterpenoid on glucose homeostasis in STZ-induced diabetic rats[J] . Journal of Physiology and Biochem- istry,2012 ,68(3) :421 - 431.
[26]MAKI K C ,CURRY L L ,REEVES M S ,et al. Chronic consumption of rebaudioside A ,a steviol glycoside ,in men and women with type 2 diabetes mellitus [ J] . Food and Chemical Toxicology ,2008 ,46 ( suppl 7) :S47-S53.
[27]RASKOVIC A ,JAKOVLJEVIC V ,MIKOV M ,et al. Joint effect of commercial preparations of Stevia rebaudiana Bertoni and sodium monoketocholate on glycemia in mice[J] . European Journal of Drug Metabolism and Pharmacokinetics ,2004 ,29(2) :83 - 86.
[28]YASUKAWA K ,KITANAKA S ,SEO S. Inhibitory effect of stevio- side on tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin [ J] . Biological & Phar- maceutical Bulletin ,2002 ,25(11) :1 488 - 1 490.
[29]SARAVANAN R , RAMACHANDRAN V. Modulating efficacy of Rebaudioside A ,a diterpenoid on antioxidant and circulatory lipids in experimental diabetic rats [ J] . Environmental Toxicology and Pharmacology,2013 ,36(2) :472 - 483.
[30]GANDHI S ,GANDHI S ,GAT Y ,et al. Natural sweeteners :Health benefits of Stevia [J] . Foods and Raw Materials ,2018 ,6 (2 ) :392 - 402.
[31] SALEHI B , LÓPEZ M D , MARTÍNEZ - LÓPEZ S ,et al ,Stevia re- baudiana Bertoni bioactive effects:From in vivo to clinical trials to- wards future therapeutic approaches [ J] . Phytotherapy Research , 2019 ,33(11) :2 904 - 2 917.
[32]PRAKASH I , MARKOSYAN A , BUNDERS C. Development of next generation Stevia sweetener : Rebaudioside M [ J] . Foods , 2014 ,3(1) :162 - 175.
[33] PURKAYASTHA S ,BHUSARI S ,PUGH G ,et al. In vitro metabo- lism of rebaudioside E under anaerobic conditions:Comparison with rebaudioside A [ J] . Regulatory Toxicology and Pharmacology , 2015 ,72(3) :646 - 657.
[34]JEPPESEN P B ,GREGERSEN S ,ALSTRUP K K ,et al. Stevioside induces antihyperglycaemic , insulinotropic and glucagonostatic effects in vivo :Studies in the diabetic Goto-Kakizaki ( GK ) rats. [J] . Phytomedicine ,2002 ,9(1) :9 - 14.
[35]JEPPESEN P B ,GREGERSEN S ,POULSEN C R ,et al. Stevioside acts directly on pancreatic beta cells to secrete insulin:Actions in- dependent of cyclic adenosine monophosphate and adenosine triphosphate-sensitive K + -channel activity [J] . Metabolism :Clini- cal and Experimental ,2000 ,49(2) :208 - 214.
[36]PANPATIL V V ,POLASA K. Assessment of stevia (Stevia rebaudi- ana) -natural sweetener:A review [J] . Journal of Food Science and Technology -Mysore- ,2008 ,45(6) :467 - 473.
[37]KARP S , WYRWISZ J , KUREK M A , et al. Combined use of co- coa dietary fibre and steviol glycosides in low-calorie muffins pro- duction[ J] . International Journal of Food Science & Technology , 2017 ,52(4) :944 - 953.
[38]OZDEMIR C ,ARSLANER A ,OZDEMIR S ,et al. The production of ice cream using Stevia as a sweetener[ J] . Journal of Food science and Technology,2015 ,52(11) :7 545 - 7 548.
[39]ALIZADEH M ,AZIZI-LALABADI M ,KHEIROURI S. Impact of u-sing stevia on physicochemical ,sensory,rheology and glycemic in- dex of soft ice cream [ J] . Food and Nutrition Sciences ,2014 ,5 (4) :390 - 396.
[40] XU Z Q ,ZHOU F ,LI X Q ,et al. Study on the technology of low sugar soybean yoghurt fermentation with Stevia glycoside[J] . Farm Products Processing ,2019 (20) :29 - 32
[41]DE OLIVEIRA B H ,PACKER J F ,CHIMLLI M ,et al. Enzymatic modification of stevioside by cell-free extract of Gibberella fujikuroi [J] . Journal of Biotechnology,2007 ,131(1) :92 - 96.
[42]MA Y Y ,CHEN Y R ,ZHANG W N ,et al. Biological conversion of stevioside to steviol by Aspergillus aculeatus and the purification of rebaudioside A[J] . Acta Microbiologica Sinica ,2014 ,54(1) :62 - 68.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






