What Are the Production Methods of Astaxanthin?
Astaxanthin is a carotenoid with high economic and practical value. It has attracted much attention due to its various physiological functions. Astaxanthin has stronger antioxidant activity than vitamin E and β-carotene [1], and can effectively inhibit oxidative damage and cancerous changes in cells [2]. It also has many other effects, such as anti-hypertension, prevention of cardiovascular disease, immune enhancement, and protection against ultraviolet radiation. In addition, astaxanthin can also be used as a food additive due to its antioxidant properties and other physiological and biochemical activities [3]. Therefore, the application of astaxanthin in the fields of medicine, food, feed, health products and cosmetics is increasing day by day.

This article describes the structural properties, sources and production methods of astaxanthin, focusing on the biosynthesis pathway of astaxanthin produced by Phafia rhodozyma, the fermentation culture conditions, and the extraction and purification methods of astaxanthin from broken cells, providing a theoretical basis for the industrial production of astaxanthin.
1 Properties and structure of astaxanthin
Astaxanthin is an unsaturated terpene compound with the chemical name 3,3'-dihydroxy-β,β'-carotene-4,4'-dione and molecular formula C40H52O4[4]. It has a melting point of 216 °C and a boiling point of 774 °C at 100 kPa[5]. Astaxanthin is hydrophobic and is easily soluble in organic solvents such as benzene, chloroform, acetone and dimethyl sulfoxide at room temperature[6], and slightly soluble in organic solvents with higher polarity such as methanol, ethanol and petroleum ether. Astaxanthin is sensitive to light, oxygen, temperature and other factors, and is prone to degradation reactions, thereby losing its biological activity.
Natural astaxanthin consists of a long carbon chain with four isoprene structures and a conjugated double bond, and a six-membered ring with α-hydroxy ketone groups at both ends. The molecular structure is similar to that of β-carotene. The two hydroxyl groups on the six-membered on the hexamer form the chiral center, which forms astaxanthin in three different configurations: levorotary (3S-3'S), dextrorotary (3R-3'R) and racemic (3R-3'S).
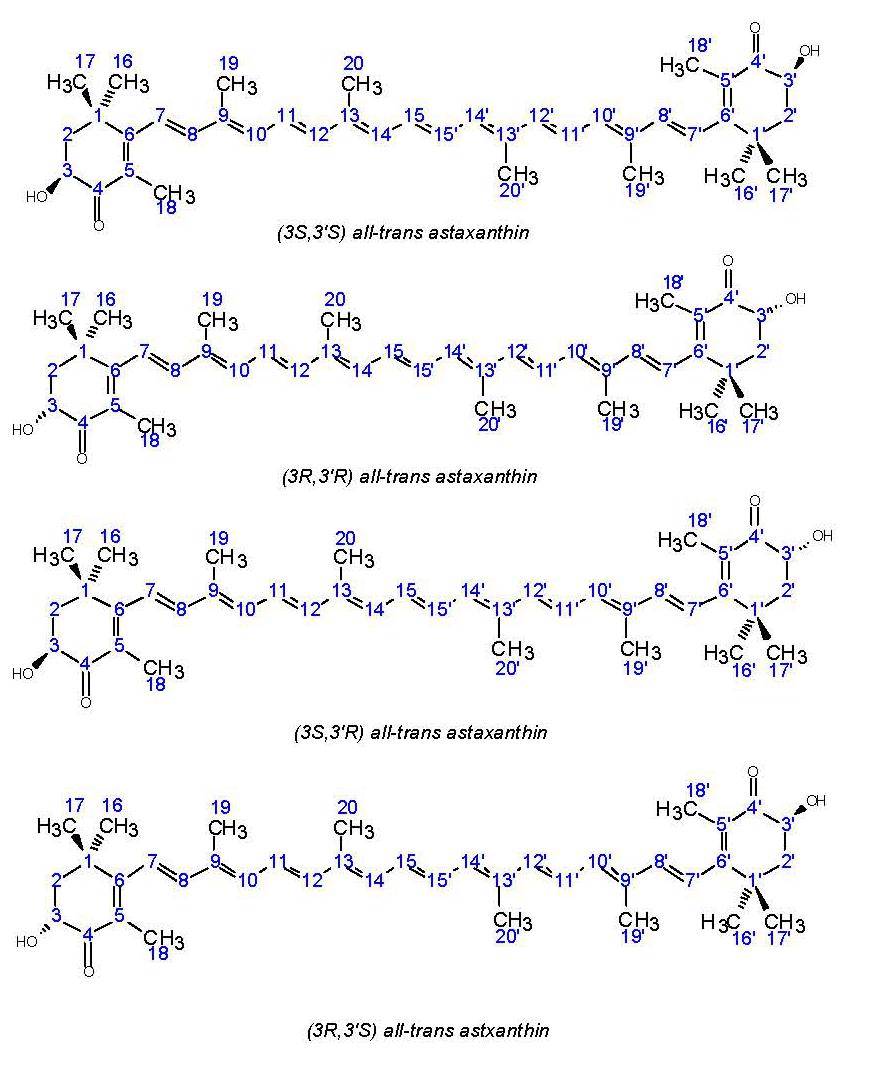
Haematococcus pluvialis contains 1.5% to 3.0% (3S-3'S) astaxanthin by dry weight, mainly in the form of astaxanthin diesters and astaxanthin monoesters[7]. In 1972, PHAFF H J[8] found that the red phaff yeast could synthesize astaxanthin and produce the dextrorotatory (3R-3'R) astaxanthin. At present, it is known that only the red phaff yeast produces natural astaxanthin with the (3R-3'R) configuration, and this natural configuration of astaxanthin has a higher bioavailability in the human body[9].
2 Natural sources and production methods of astaxanthin
2.1 Natural sources
Astaxanthin is widely found in animals (such as aquatic animals and birds), plants, fungi, algae and bacteria. Wild salmon obtain astaxanthin from the food chain, but farmed salmon obtain the characteristic colour of their flesh from astaxanthin-containing feed [10]. The brilliant color of flamingo feathers is also due to the presence of astaxanthin. The content and state of astaxanthin in animals vary. For example, the astaxanthin in the muscles, internal organs and plasma of salmon is mainly in the free state, while the astaxanthin in the skin, scales and roe is mainly in the esterified form. No animal can synthesize astaxanthin from scratch, and it needs to be obtained from algae, yeast, and plants [11]. At present, astaxanthin is widely used, and consumer demand is constantly increasing. Relying solely on astaxanthin present in the food chain is insufficient to meet the needs of various industries. Existing astaxanthin production methods mainly include chemical synthesis, natural extraction, and biosynthesis.

2.2 Production methods
2.2.1 Chemical synthesis
The chemical synthesis method refers to the production of astaxanthin using multi-step chemical and biocatalytic reactions. According to the differences in the synthesis method, the chemical synthesis method is divided into the semi-synthesis method and the total synthesis method. The semi-synthesis method refers to the method of preparing astaxanthin using precursor substances (such as lutein and canthaxanthin) in the astaxanthin metabolic pathway as raw materials; the total synthesis method refers to the method of obtaining astaxanthin completely using chemical synthesis [12].
Chemically synthesized astaxanthin has the advantages of low production cost, high yield, and astaxanthin purity of more than 96% [13]. However, chemically synthesized astaxanthin is a mixture of various conformations and contains by-products, and its absorption and utilization rate in the body is low [14]. Its stability, safety, and antioxidant activity are lower than those of naturally extracted astaxanthin [15].
2.2.2 Natural extraction method
Natural astaxanthin is mostly found in marine organisms. The method of extracting astaxanthin by crushing shrimp, crab and other processed by-products rich in astaxanthin, removing lime, and using organic solvents is called the natural extraction method. This preparation method can promote the development of aquaculture while reducing the environmental pollution caused by the waste by-products of aquatic products. However, because the discarded shells of shrimp and crab have high ash and chitin content, and the low astaxanthin content makes the extraction process complicated, there are problems with high extraction costs [16].
2.2.3 Microbial fermentation method
The method of using yeast, algae and bacteria to produce astaxanthin is called the microbial fermentation method. The main strains include the single-cell green algae Haematococcus pluvialis, Chlorella aeruginosa [11], Rhodotorula rubra, Rhodotorula glutinosa [18] and Paracoccus [19-20]. Fermentation-based production of astaxanthin has a clear structure, few by-products and is environmentally friendly. However, it is constrained by factors such as low yield, strict culture conditions and high culture costs. The use of cheap culture materials and the selection and breeding of high-quality, high-yielding strains to enable industrial production are key factors in the microbial fermentation-based production of astaxanthin.
3 Astaxanthin-producing microorganisms
3.1 Algae producing astaxanthin
Many algae can produce astaxanthin, such as Haematococcus pluvialis, Chlamydomonas, Acetabularia, Euglena, etc. Haematococcus pluvialis is a freshwater single-cell green alga belonging to the Chlorophyta, Chlorophyceae, Haematococcus genus, and is the main astaxanthin-producing algae. The astaxanthin in Haematococcus pluvialis cells mainly exists as diesterified astaxanthin and monoesterified astaxanthin, with a small amount in the free state. However, Haematococcus pluvialis has a long growth time, strict culture conditions, requires light, has limited production sites, and astaxanthin is found in the thick-walled spores, which have a low extraction rate and poor continuity [21-23]. In 2010, the Ministry of Health approved Haematococcus pluvialis as a new source of food. Since then, a variety of health foods rich in Haematococcus pluvialis astaxanthin have been approved by the State Food and Drug Administration. These measures have had a positive impact on promoting the research and development of astaxanthin products and the rapid development of the industry [24].
Chlorella pyrenoidosa is another green algae that produces natural astaxanthin. The astaxanthin content of Chlorella pyrenoidosa is lower than that of Haematococcus pluvialis, but this algae has special advantages for cultivation. Chlorella pyrenoidosa is an aerobic heterotroph that can use glucose as the sole carbon source. It grows quickly, can reach ultra-high cell densities, is less sensitive to adverse environmental conditions, and is easy to cultivate indoors and outdoors.
3.2 Bacteria producing astaxanthin
Astaxanthin is found in a variety of bacteria such as Brevibacterium, Corynebacterium, and Mycobacterium lacticola. Although the astaxanthin content of most bacteria is much lower than that of algae and Rhodotorula glutinis [25-27], the problem of low astaxanthin production in bacteria can be improved by introducing astaxanthin synthesis-related genes into the bacteria. In particular, Gram-negative bacteria [28] have thin and easily broken cell walls, making it easy to extract pigments, and are suitable for large-scale high-density fermentation cultures [11]. The production of astaxanthin by bacterial fermentation can greatly reduce the production cost of natural astaxanthin and is of great significance for the future industrial production of astaxanthin.
3.3 Yeast production of astaxanthin
The main strains used in astaxanthin production by yeast fermentation include Rhodotorula glutinis, Rhodotorula rubra[29], Rhodotorula benthica[30-31] and Rhodotrula glutinis.
Red fife yeast is the only species in the kingdom of fungi, phylum of fungi, subphylum of imperfect fungi, family of cryptococcus, genus of red fife yeast. It reproduces by budding during asexual reproduction and metabolizes by both aerobic respiration and anaerobic respiration. It is currently a commonly used fungus for microbial fermentation to produce astaxanthin at home and abroad [32-34]. The astaxanthin content of the wild strain of Rhodotorula fava is 0.05% of the dry cell mass, and some mutant strains can reach 1.0%, accounting for about 80% of the total carotenoid content. Red yeast fermentation has the following advantages in the production of astaxanthin: it can use a variety of carbon and nitrogen sources to produce astaxanthin, and the cells grow and multiply rapidly, allowing for high-density cultivation; the production cycle is short and the cost is low; the cell walls are easily broken, and the astaxanthin produced is in the dextrorotatory configuration (3R-3'R) and is in a free state, which is easily absorbed by the human body. After extraction, the yeast cell body can be directly used as a feed additive [4, 35].
4 Biosynthesis of astaxanthin by yeast
Many studies have shown that mevalonate (MVA), isopentenyl pyrophosphate (IPP), farnesyl pyrophosphate (FPP), dimethylallylpy-rophosphate , geranyl-geranyl pyrophosphate (GGPP), octahydro-lycopene, tetrahydro-lycopene, β-carotene, etc. are important intermediates in the biosynthesis pathway of astaxanthin. The biosynthetic pathway of astaxanthin in yeast is divided into two stages: the first stage is the synthesis of β-carotene; the second stage is the production of astaxanthin from β-carotene through oxidation and hydroxylation [36].
The carotenoid in yeast is derived from the methionine pathway, starting from glucose, through the glycolysis pathway (embden-meyerhof pathway, EMP) to produce pyruvate, and then oxidized and decarboxylated to obtain acetyl coenzyme A (Acetyl-CoA), and three three molecules of Acetyl-CoA condense to form MVA, which is then converted by phosphorylation and decarboxylation into isopentenyl pyrophosphate (C5). IPP is the synthetic precursor of all isopentenyl compounds (such as astaxanthin, carotene and ergosterol). IPP is condensed to form GGPP (C20), and two molecules of GGPP undergo dimerization to form the colorless octahydro-tomato red pigment, which is considered to be the first specific step in carotenoid synthesis. This is followed by a multi-step dehydrogenation and a one-step cyclization to synthesize β-carotene [37]. Finally, astaxanthin is produced from β-carotene through a two-step enzymatic reaction, in which ketolase catalyzes the introduction of two ketone groups at the 4 position and hydroxylase catalyzes the introduction of two hydroxyl groups at the 3 position of the β-carotene molecule.
5 Control and optimization of the yeast fermentation process
The astaxanthin-producing yeast has a high metabolic capacity and can utilize monosaccharides [38], disaccharides and polysaccharides, organic acids and alcohols. It can also rapidly utilize simple nitrogen sources such as ammonium, nitrate, urea or amino acids, as well as complex mixtures such as yeast extract, beef extract, malt extract or peptone. It can also utilize industrial waste materials, which can effectively reduce production costs, such as waste from the sugar production process, wet corn milling process [39] or enzymatic hydrolysis solutions of wood [40]. STOKLOSA R J et al. [41] used sweet sorghum bagasse (SSB) in a 2 L fermenter with Pichia pastoris to produce 65.4 mg/L astaxanthin, which accounted for 2.49 mg/g of total astaxanthin. However, low-cost media may contain unknown inhibitors of carotene production, making them unsuitable for the production process [42].
Fermentation of astaxanthin in the presence of SSB hydrolysate based on the above experiments instead reduced the astaxanthin content to 53.3 mg/L, which may be due to the inhibitory effect of phenolic compounds in SSB [27, 41]. In most cases, the culture medium must be supplemented with the necessary nutrients, and it may also include inducers or precursors of carotene production [43]. The addition of some ingredients can increase the production of astaxanthin, such as the addition of fresh tomato juice [44] and carrot juice [45].
Nutrients (carbon sources, nitrogen sources, metal ions, vitamins, etc.) and physical factors (temperature, pH, oxygen supply, etc.) can affect cell growth and astaxanthin production. The different strains used in the literature or the high-yielding mutant strains of astaxanthin have resulted in different compositions of the culture medium and fermentation process conditions (see Table 1). At the same time, the mutant strains contain randomly inserted gene fragments, and the quantity or location and the exact functional nature of the inserted genes are still unknown in most cases, which may affect the comparability between the literature. The effects of nutrients and culture methods on cell growth and astaxanthin production can be summarized as follows.
Red yeast is a mesophilic yeast with a growth temperature range of 0–27 °C. Depending on the (mutant) strain used, the optimum temperature for astaxanthin production and yeast cell growth is usually between 18 and 22 °C. The optimum pH for astaxanthin synthesis and yeast cell growth is usually between 5 and 6. The optimal temperature or pH for yeast cell growth is generally different from the optimal temperature or pH for astaxanthin synthesis and accumulation. Therefore, changing the pH or temperature during fermentation can increase astaxanthin production during the fermentation process. Both mutant and wild strains, the culture temperature and the pH of the culture medium have a strong effect on the astaxanthin content and carotenoid composition of the cells [52-53].
Oxygen plays a key role in astaxanthin biosynthesis, and the amount of astaxanthin accumulated is related to the rate of oxygen transfer. Insufficient oxygen supply leads to the accumulation of β-carotene and reduces the efficiency of β-carotene oxidation to astaxanthin. Therefore, sufficient oxygen can help with the accumulation of astaxanthin [54]. There is a critical dissolved oxygen concentration at 10% to 20% air saturation, below which dissolved oxygen concentration inhibits cell growth and carotenoid formation.
However, excessive oxygen content inhibits yeast cell growth. Therefore, providing the right amount of oxygen to the red fife yeast cells can help to improve astaxanthin synthesis. Therefore, the oxygen consumption of the bacteria needs to be determined when cultivating different strains in order to adjust the speed of the fermentation equipment. In addition to adjusting the speed and changing the air flow rate to increase oxygenation, adding biocompatible organic solvents with high oxygen solubility to the culture medium as oxygen carriers, such as oleic acid, n-dodecane, soybean oil, Tween-80, and ethyl acetate, can also improve the oxygen transfer rate of the cells.
During the exponential growth phase, high sugar concentrations inhibit the two processes of lycopene synthesis β-carotene and β-carotene synthesis astaxanthin. Therefore, high carbon source concentrations should not be used [54]. However, in the later stages of cell growth, high carbon source concentrations can promote the accumulation of carotenoids [55]. Therefore, the antagonistic effect of high sugar concentrations can be eliminated by using a batch feeding process, while achieving high biomass and high intracellular astaxanthin concentrations.
In industry, the fermentation process of Rhodotorula glutinis is divided into two stages: the cell growth stage and the maturation stage. By providing a low C/N ratio (the ratio of carbon source to nitrogen source concentration), the cells initially exhibit rapid growth, and the growth rate gradually slows down as the high cell concentration is approached. During this phase, the growth rate of the cells is higher than the formation rate of astaxanthin. When the cells are close to the stable growth period, the conversion to a high carbon-to-nitrogen ratio is switched, and the astaxanthin synthesis rate is higher than the cell growth rate. Within the same fermentation time, by regulating the carbon source concentration and carbon-to-nitrogen ratio at different stages of bacterial fermentation, both high cell yield and high astaxanthin yield can be achieved simultaneously.
The (mutant) strain and medium used determine the feeding control to achieve maximum process productivity and can be established in several ways. Examples are feeding based on the Monod index, pH-stat control [40], DO-stat culture control or pulsed feeding after determining the concentration of the carbon source (see Table 1). As an alternative to the fed-batch process, semi-continuous and continuous processes can be considered. Evaluating the data in Table 1, pulse feeding and fed-batch feeding seem to be better feeding methods. Another way to increase astaxanthin during the maturation phase is by adding slowly metabolized carbon sources such as glycerol or acetic acid after the initial metabolic carbon source is depleted.
6 Purification methods for astaxanthin from different sources
6.1 Methods for breaking the cell wall of astaxanthin
Astaxanthin is an intracellular product, and generally needs to go through steps such as cell wall breaking, extraction and purification before it can be extracted from yeast cells. Commonly used cell wall breaking methods include mechanical methods, chemical methods [56], enzymatic methods and heat treatment [57].
Mechanical methods use mechanical equipment to tear the cell walls and release the contents through osmotic pressure inside the cells. The main methods are ultrasonic crushing, bead milling, spray impact crushing, and high-pressure homogenization. Mechanical methods are widely used because they are easy to operate, but they can easily cause the solution temperature to rise in some places, resulting in astaxanthin loss.
Chemical methods mainly include the dimethyl sulfoxide method, the acid-base heating method, and organic solvent permeation. The alkali extraction method and acid hydrolysis method require the consumption of large amounts of alkali and organic acids to break the walls, which increases the amount of sewage discharged, causing environmental pollution. In addition, strong acids and bases can damage astaxanthin. Using a 5.55 mol/L lactic acid concentration and a 30 ℃ crushing temperature for wall breaking and extraction can reduce the damage to astaxanthin. The final extracted astaxanthin and total carotenoid contents were 1 294.7 μg/g and 1 516.0 μg/g, respectively, and astaxanthin accounted for 85.4% of the total extract [56].
β-glucanase and snail enzymes can hydrolyze the cell wall skeleton component β-glucan, which can break the cell wall more effectively than other methods and avoid the loss of astaxanthin due to leakage from the cell. Enzyme treatment has mild conditions, low equipment requirements, and the treatment process causes less environmental pollution. The extracted astaxanthin is also more stable than that obtained by other methods.
At present, a variety of modern extraction methods have been developed for extracting active ingredients, such as pulsed electric field (PEF) [58], high-pressure microfluidisation (HPMF), ionic liquids (ionic liquids, ILs) [59] and other emerging technologies. The application of PEF may cause lethal damage to cells or induce sublethal stress through transient permeabilization of cell membranes and electrophoretic movement of charged substances between cell compartments. Some scholars have studied the use of PEF to extract different valuable compounds from microalgae.
HPMF is an emerging technology for high-speed impact, strong shearing, transient pressure drops, high-frequency vibrations, cavitation and ultra-high pressures (up to 200 MPa) in emulsions, macromolecular modification and the extraction of bioactive ingredients. Compared to conventional high-pressure homogenization, HPMF has a different valve and chamber design and a higher operating pressure. ILs consist of cations and anions that are held loosely together and are characterized by negligible vapor pressure, low melting temperature, excellent thermal and chemical stability.
In addition, they have a high capacity for dissolving cellulose, and mixtures of ionic liquids have a low effect on the lipid extraction of chlorella. Therefore, ILs are a novel cell disruption technique that can be used to recover lipids and proteins from chlorella. The efficiency of cell wall disruption for the extraction of astaxanthin from Haematococcus pluvialis was compared using various techniques such as PEF, ultrasound (US), HPMF, HCl and ILs. The results showed that ILs, HCl and HPMF treatments were the most effective in cell disruption, with an astaxanthin extraction rate of over 80%, while PEF and US were less effective in cell wall disruption [60]. Compared with traditional cell disruption techniques, emerging cell disruption techniques such as PEF, HPMF and ILs have less impact on astaxanthin. They also use less solvent, are time-saving, energy-saving and environmentally-friendly.
6.2 Astaxanthin extraction methods
Astaxanthin is a fat-soluble substance that is soluble in organic solvents but not in water. It can be extracted using polar organic solvents such as acetone, ethanol, methanol, and petroleum ether. The results of the effect of different solvents on the extraction rate of total carotenoids from Antarctic krill show that anhydrous ethanol has the best extraction effect, with a total carotenoid extraction rate of 73.3% [61]. However, astaxanthin is soluble in organic solvents but has a low solubility, so the effect of a single solvent extraction is limited. Huang Kaichen et al. [62] used a 2:1 mixture of ethyl acetate and ethanol as the extraction solution, and the astaxanthin content extracted by acid heating was significantly higher than that of a single solution.
6.3 Purification and detection methods for astaxanthin
In terms of astaxanthin purification, thin layer chromatography (TLC) and column chromatography are mainly used. Thin layer chromatography can be used to simply determine the composition of crude extracts. However, this method has low resolution, poor reproducibility, is easily affected by external factors, places high requirements on the operator, and is not conducive to post-purification experimental operations. Compared with other purification methods, column chromatography is the most commonly used method because it is inexpensive and convenient to replace the stationary and mobile phases. The combination of different stationary and mobile phases can achieve the separation and purification of relatively simple samples, and has a wide range of applications.
Thin-layer chromatography and column chromatography are suitable for preliminary purification. Later purification can be carried out using high performance liquid chromatography (HPLC), which can achieve a purification effect of over 98%, but the preparation cost is high. HPLC can not only obtain high-purity astaxanthin, but also accurately determine the astaxanthin content using a suitable mobile phase and a C18 or C30 high performance liquid chromatography column. In experiments, the UV-Vis spectrophotometry method is often used to quickly determine the amount of astaxanthin produced.
7 Conclusion and outlook
Astaxanthin has broad development potential and is of great value and has room for development in medicine, cosmetics, health products, feed additives, and other fields. Both the natural astaxanthin and the chemically synthesized astaxanthin preparation processes have certain disadvantages. In the future, research on the microbial synthesis of astaxanthin will focus on developing high-yielding strains with stable genetic traits, using low-cost culture materials, exploring simple production processes, and using advanced, rapid and precise extraction and purification techniques to reduce production costs and improve astaxanthin yield and purity.
Reference:
[1] SHAH M M, LIANG Y, CHENG J J, et al, et al. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products[J]. Front Plant Sci, 2016, 7: 531.
[2] FARAONE I, SINISGALLI C, OSTUNI A, et al. Astaxanthin anticancer ef- fects are mediated through multiple molecular mechanisms: A systematic re- view[J]. Pharmacol Res, 2020, 155: 104689.
[3] FAKHRI S, ABBASZADEH F, DARGAHI L, et al. Astaxanthin: A mecha- nistic review on its biological activities and health benefits [J]. Pharmacol Res, 2018, 136: 1-20.
[4] Cai Jun, You Zhineng. Research progress in the production of astaxanthin by fermentation [J]. Food Science, 2015, 36 (23): 358-366.
[5] Zhang Xiaona, Hu Baodi, Pei Lingpeng, et al. Research overview of the functional factor astaxanthin [J]. China Food Additives, 2017 (8): 208-214.
[6] Zhou Pingping. Research on astaxanthin biosynthesis and metabolic regulation in Saccharomyces cerevisiae [D]. Hangzhou: Zhejiang University, 2018.
[7] LI X, WANG X Q, DUAN C L, et al. Biotechnological production of astax- anthin from the microalga Haematococcus pluvialis[J]. Biotechnol Adv, 2020, 43: 107602.
[8] PHAFF H J. A comparative study of the yeast florae associated with trees of the Japanese Islands and on the West Coast of North America[J]. Ferment Technol Today, 1972: 759-774.
[9] RAMÍREZ J, NUÑEZ M L, VALDIVIA R. Increased astaxanthin production by a Phafia rhodozyma mutant grown on date juice from Yucca fillifera[J]. J Ind Microbiol Biot, 2000, 24(3): 187-190.
[10] EDWARDS JA, BELLION P, BEILSTEIN P, et al. Review of genotoxicity and rat carcinogenicity investigations with astaxanthin[J]. Regul Toxicol Pharm, 2016, 75: 5-19.
[11] FANG N, WANG C, LIU X, et al. De novo synthesis of astaxanthin: From organisms to genes[J]. Trends Food Sci Tech, 2019, 92: 162-171.
[12] Xian Qizhi, Lin Jindong, Zhou Yingfang, et al. Research on the thermal isomerization of astaxanthin converted from natural lutein [J]. China Food Additives, 2019, 30(3): 87-93.
[13] Wang Duoren. Progress in the development and application of astaxanthin [J]. Jiangxi Food Industry, 2011(3): 38-41.
[14] ERNST H. Recent advances in industrial carotenoid synthesis[J]. Pure Appl Chem, 2013, 74(11): 1369-1382.
[15] Cui H. Biological functions of astaxanthin and its application in animal production [J]. Feed Research, 2019, 42(9): 112-115.
[16] BON J A, LEATHERS T D, JAYASWAL R K. Isolation of astaxanthin- overproducing mutants of Phafia rhodozyma[J]. Biotechnol Lett, 1997, 19(2): 109-112.
[17] ZHUANG Y, JIANG G L, ZHU M J. Atmospheric and room temperature plasma mutagenesis and astaxanthin production from sugarcane bagasse hydrolysate by Phafia rhodozyma mutant Y1[J]. Process Biochem, 2020, 91: 330-338.
[18] LEYTON A, FLORES L, MAKI-ARVELA P, et al. Macrocystis pyrifera source of nutrients for the production of carotenoids by a marine yeast Rhodotorulamucilaginosa[J]. J Appl Microbiol, 2019, 127(4): 1069-1079.
[19] ASKER D, AWAD T S, BEPPU T, et al. Purification and identification of astaxanthin and its novel derivative produced by radio-tolerant Sphin- gomonas astaxanthinifaciens[J]. Method Mol Biol, 2018, 1852: 171-192.
[20] HENKE N A, HEIDER S A E, PETERS W P, et al. Production ofthe ma- rine carotenoid astaxanthin by metabolically engineered Corynebacterium glutamicum[J]. Mar Drug, 2016, 14(7): 124.
[21] IP P F, WONG K H, FENG C. Enhanced production of astaxanthin by the green microalga Chlorelazofingiensis in mixotrophic culture[J]. Process Biochem, 2003, 39(11): 1761-1766.
[22] KATSUDA T, SHIRAISHI H, ISHIZU N, et al. Effect of light intensity and frequency of flashing light from blue light emitting diodes on astaxan- thin production by Haematococcus pluvialis[J]. J Biosci Bioeng, 2008, 105(3): 216-220.
[23] KIM Z H, KIM S H, LEE H S, et al. Enhanced production of astaxanthin by flashing light using Haematococcus pluvialis[J]. Enzyme Microb Tech, 2006, 39(3): 414-419.
[24] Peng Y, Ren X, Chen L, et al. Research progress on the technology of astaxanthin preparations and its effect on the stability of astaxanthin [J]. China Oil and Fat, 2019, 44(4): 115-121.
[25] JEEVANANTHAM G, VINOTH M, HUSSAIN J M, et al. Biochemical characterization of five marine cyanobacteria species for their biotechnolog-ical applications[J]. J Pharmacognosy Phytochem, 2019, 8(2): 635-640.
[26] KHOO K S, LEE S Y, OOI C W, et al. Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis[J]. Bioresource Technol, 2019, 288: 121606.
[27] STOKLOSA R J, JOHNSTON D B, NGHIEM N P. Phafia rhodozyma cultivation on structural and non-structural sugars from sweet sorghum for astaxanthin generation[J]. Process Biochem, 2019, 83: 9-17.
[28] Zhu Y. Research on the process of astaxanthin fermentation by Rhodotorula glutinis [D]. Wuhan: Huazhong University of Science and Technology, 2007.
[29] Luo X Z, Lin Y Y, Chen Y J, et al. Optimization of fermentation conditions for astaxanthin production by Rhodotorula glutinis RG-31 [J]. Fujian Agricultural Science and Technology, 2019 (9): 16-21.
[30] Wang Fuqiang, Zhang Aiqing, Liu Xifeng, et al. Research progress on the process of activating marine red yeast to cultivate astaxanthin [J]. Modern Food, 2018 (20): 149-150, 162.
[31] Zhao Di. Research on the optimization of marine red yeast culture conditions and the improvement of astaxanthin yield [D]. Dalian: Dalian Polytechnic University, 2015.
[32] ANARJAN N, TAN C P. Developing a three component stabilizer system for producing astaxanthin nanodispersions[J]. Food Hydrocolloid, 2013, 30(1): 437-447.
[33] WANG J, HAN D, SOMMERFELD M R, et al. Effect of initial biomass density on growth and astaxanthin production of Haematococcus pluvialis in an outdoor photobioreactor[J]. J Appl Phycol, 2013, 25(1): 253-260.
[34] YUAN C, JIN Z Y, XU X M, et al. Inclusion complex of astaxanthin with hydroxypropyl-β-cyclodextrin: UV, FTIR, 1H NMR and molecular model- ing studies[J]. Carbohyd Polym, 2012, 89(2): 492-496.
[35] Peng Xiaolan. Research on the physiological function, production and application of astaxanthin [J]. Contemporary Animal Husbandry, 2005 (11): 50-52.
[36] Xu Cairong. Research on measures to increase astaxanthin production [D]. Tai'an: Shandong Agricultural University, 2013.
[37] Chi S. Strategies for improving astaxanthin synthesis by Pichia pastoris and analysis of metabolic regulation characteristics [D]. Beijing: China Agricultural University, 2016.
[38] Xiao A, Yu G, Cai H, et al. Strategies for pH control of Pichia pastoris fermentation and their effects on astaxanthin synthesis [J]. Chinese Journal of Food Science, 2015, 15(1): 66-72.
[39] Zhu Xiaoli, Liang Shizhong, Deng Maocheng, et al. pH-controlled fed-batch cultivation of Rhodotorula glutinis for astaxanthin production [J]. Food Research and Development, 2011, 32(4): 160-164.
[40] VÁZQUEZ M, MARTIN A M. Optimization of Phafia rhodozymacontin- uous culture through response surface methodology[J]. Biotechnol Bioeng, 2015, 57(3): 314-320.
[41] STOKLOSA R J, JOHNSTON D B, NGHIEM N P. Utilization of sweet sorghum juice for the production of astaxanthin as a biorefinery co-product by Phafia rhodozyma[J]. ACS Sust Chem Eng, 2018, 6(3): 3124-3134.
[42] OKAGBUE R N, LEWIS M J. Use of alfalfa residual juice as a substrate for propagation of the red yeast Phafia rhodozyma[J]. Appl Microbiol Biot, 1984, 20(1): 33-39.
[43] CALO P, MIGUEL T, VELÁZQUEZ J B, et al. Mevalonic acid increases trans-astaxanthin and carotenoid biosynthesis in Phafia rhodozyma [J]. Biotech Lett, 1995, 17(6): 575-578.
[44] Zhu Xiaoli, Liang Shizhong. Study on the cultivation of red yeast for astaxanthin production in 50 L and 500 L reactors [J]. Modern Food, 2016 (13): 106-110.
[45] Hu Xiangdong, Pan Lingyan, Ye Mao, et al. Selection of high astaxanthin-producing Rhodotorula strains and optimization of fermentation parameters [J]. Food Industry Science and Technology, 2016, 37 (5): 142-147.
[46] Song Chao. Application of metabolic network in astaxanthin biosynthesis [D]. Dalian: Dalian Polytechnic University, 2010.
[47] Zhu Xiaoli, et al. pH-stat controlled fed-batch cultivation of Rhodotorula glutinis for astaxanthin production [J]. Food Research and Development, 2011, 32(4): 160-164.
[48] Jin Jin. Single-base mutation evolution and genome rearrangement of astaxanthin-producing Saccharomyces cerevisiae [D]. Tianjin: Tianjin University, 2018.
[49] Jiang Xinglong, Hong Qinglin, Cai Huinong, et al. Effect of feeding process on astaxanthin production by two strains of Pichia pastoris [J]. Chinese Journal of Microbiology, 2013, 40(11): 1996-2004.
[50] Fu Shuang, Shen Ningyan, Ni Hui, et al. The effect of ethanol feeding on the promotion of astaxanthin production by Pichia pastoris fermentation [J]. Journal of Jimei University (Natural Science Edition), 2017, 22(4): 20-27.
[51] LIU Z Q, ZHANG J F, ZHENG Y G, et al. Improvement of astaxanthin production by a newly isolated Phafia rhodozyma mutant with low-energy ion beam implantation[J]. J Appl Microbiol, 2008, 104(3): 861-872.
[52] RAMIÍREZ J, GUTIERREZ H, GSCHAEDLER A. Optimization of astaxan- thin production by Phafia rhodozyma through factorial design and response surface methodology[J]. J Biotechnol, 2001, 88(3): 259-268.
[53] FRENGOVA G I, BESHKOVA D M. Carotenoids from Rhodotorula and Phafia: yeasts of biotechnological importance[J]. J Ind Microbiol Biot, 2009, 36(2): 163-180.
[54] MIAO L, CHI S, WU M, et al. Deregulation of phytoene-β-carotene syn- thase results in derepression of astaxanthin synthesis at high glucose con- centration in Phafia rhodozymaastaxanthin-overproducing strain MK19[J]. BMC Microbiol, 2019, 19(1): 133.
[55] MEYER P S, PREEZ J C. Astaxanthin production by a Phafia rhodozyma mutant on grape juice[J]. World J Microb Biot, 1994, 10(2): 178-183.
[56] NI H, CHEN Q H, HE G Q, et al. Optimization of acidic extraction of as- taxanthin from Phafia rhodozyma[J]. J Zhejiang U Sci B, 2008, 9(1): 51-59.
[57] Teng Changying, Zhang Li, Qin Song. Physiological functions, biosafety and application potential of astaxanthin [J]. Hubei Agricultural Sciences, 2006 (6): 827-829.
[58] MARTÍNEZ J M, SCHOTTROFF F, HAAS K, et al. Evaluation of pulsed electric fields technology for the improvement of subsequent carotenoid ex- traction from dried Rhodotorula glutinis yeast[J]. Food Chem, 2020, 323: 126824.
[59] GAO J, FANG C L, LIN Y Z, et al. Enhanced extraction of astaxanthin us- ing aqueous biphasic systems composed of ionic liquids and potassium phosphate[J]. Food Chem, 2020, 309: 125672.
[60] LIU Z W, ZENG X A, CHENG J H, et al. The efficiency and comparison of novel techniques for cell wall disruption in astaxanthin extraction from Haematococcus pluvialis[J]. Int J Food Sci Technol, 2018, 53(9): 2212- 2219.
[61] Song, Sumei. Extraction, isolation and purification of astaxanthin from Antarctic krill shells [D]. Wuxi: Jiangnan University, 2013.
[62] Huang Kaishen, Liao Zhiying, Xu Chunhou, et al. Optimization of the extraction and separation and purification of astaxanthin from the yeast Schizochytrium sp. J. Natural Product Research and Development, 2018, 30 (11): 1858-1862, 1877.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese