What Are the Benefits of Centella Asiatica?
Centella asiatica is the dried whole herb of the plant Centella asiatica (L.) Urb. in the family Apiaceae. It has the effects of clearing away heat and dampness, detoxifying and reducing swelling. It is mainly used to treat damp-heat jaundice, heatstroke diarrhea, stone dysuria, hematuria, carbuncle and swelling caused by toxin, bruises and contusions, etc.[1]. When previous scholars analyzed the chemical composition of Centella asiatica, they found that its main components include triterpenoids, essential oils, polyacetylenes, flavonoids, etc., and triterpenoids are the most abundant [2]. Madecassoside is a triterpenoid compound found in Centella asiatica. Its molecular formula is C48H78O19 and molecular weight is 959.12[3]. As one of the main pharmacologically active ingredients in Centella asiatica, madecassoside has a variety of pharmacological effects, such as anti-tumor, anti-inflammatory, protecting the nervous system, and promoting wound healing[4].

1. Anti-tumor effect and mechanism of Centella asiatica extract
1.1. Inhibit the proliferation of tumor cells
Zhou et al. [5] showed that asiaticoside can inhibit the proliferation of colorectal cancer cells by inhibiting the nuclear transcription factor κB (NF-κB) signaling pathway. In addition, asiaticoside can also inhibit the proliferation of pancreatic cancer cells by inhibiting the phosphorylation of p38 mitogen-activated protein kinase (p38MAPK) and NF-κB p65 [6].
1.2 Inducing apoptosis in tumor cells
Zeng Manhong et al. [7] used different concentrations of asiaticoside to treat gastric cancer SGC-7901 cells, and the cells showed a significant apoptotic peak. Transmission electron microscopy revealed typical characteristics of apoptosis, such as cytoplasmic concentration, nuclear shrinkage and rupture, and mitochondrial aggregation. Centella asiatica extract also promotes apoptosis in breast cancer MCF-7 cells: as the intervention time of centella asiatica extract is prolonged, the morphology of MCF-7 cells becomes irregular, the cytoplasm gradually spreads, the nuclei first fuse and then gradually disappear, eventually forming apoptotic bodies. Further research found that asiaticoside can significantly reduce the expression levels of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) proteins in MCF-7 cells [8]. Wu Qian et al. [9] found that different concentrations of asiaticoside can induce apoptosis in osteosarcoma Saos-2 cells, and can reduce the expression levels of phosphatidylinositol 3-kinase (PI3K), protein kinase B (Akt), phosphorylated Akt (p-Akt), glycogen synthase kinase 3β (GSK-3β), phosphorylated glycogen synthase kinase 3β (p-GSK-3β), indicating that asiaticoside can induce apoptosis in osteosarcoma Saos-2 cells by regulating the activity of the PI3K/Akt/GSK-3β signaling pathway. Other studies have also shown that asiaticoside can induce apoptosis in liver cancer QGY-7703 and Bel-7402 cells by inhibiting the activity of the PI3K/Akt and MAPK/extracellular signal-regulated kinase (ERK) signal pathways [10].
Bax, a Bcl-2-related X protein, belongs to the Bcl-2 family and is the main apoptosis regulator in the human body [11]. Pan Jinli [12] found that the induction of apoptosis in A2780 ovarian cancer cells by asiaticoside may be related to the downregulation of the expression of the anti-apoptotic factor Bcl-2 and the upregulation of the pro-apoptotic factor Bax. Related studies have shown that after 48 hours of acting on breast cancer MCF-7 cells, asiaticoside significantly induces apoptosis in the cells, and the expression level of caspase-3 (c-asiaticoside p-asiaticoside e-3) in the cells is significantly increased, tumor necrosis factor (TNF-α) and interleukin 1β (IL-1β) were significantly reduced [13-14]. In addition, Li et al. [15] found that asiaticoside can exert antitumor effects on the drug-resistant myeloma cell line KM3/BTZ cells. The mechanism is related to inducing autophagy, activating the c-asiaticoside p-asiaticoside e-3 activity, and inhibiting cell migration, invasion, and the signal transducer and activator of transcription 3 (STAT-3) signaling pathway. Thus, asiaticoside can induce apoptosis in tumor cells by regulating the expression of apoptosis-related proteins and mediating apoptosis-related signal pathways (such as the PI3K/Akt/GSK-3β signal pathway), thereby inhibiting the proliferation, migration, and invasion of tumor cells.
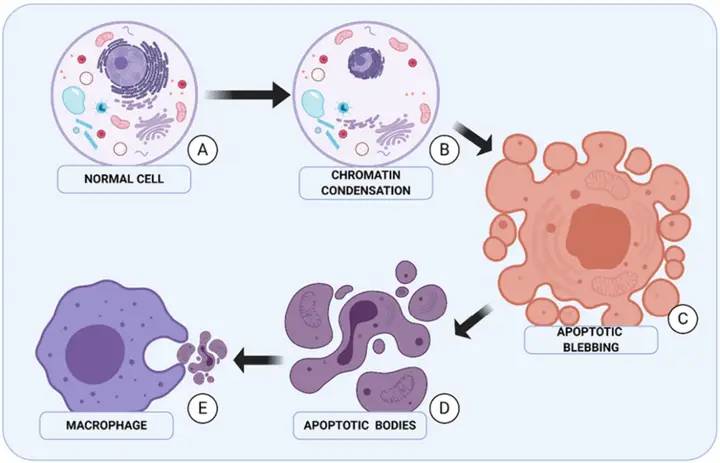
2. Anti-fibrosis effect and mechanism of asiaticoside
Related studies have found that when the mass concentration of asiaticoside is 400 μg/mL, it exhibits inhibitory effects on human embryonic skin fibroblasts and human lung fibroblasts [16-17]. In a rat lung mesenchymal fibrosis experiment induced by pimecrolimus, it was found that asiaticoside improved pimecrolimus-induced lung mesenchymal fibrosis by upregulating the expression level of adenosine 2A receptors (A2AR) and downregulating the expression levels of IL-4, TNF-α and transforming growth factor beta 1 (TGF-β1) [18]. Zhu Dewei et al. [19] found that asiaticoside can inhibit the production of inflammatory cytokines, reduce the expression level of TGF-β1 mRNA, and exert an anti-fibrotic effect.
Wang Ce [20-21] found that asiaticoside can significantly improve renal fibrosis in rats with unilateral ureteral obstruction. The mechanism may be related to down-regulating the expression of connective tissue growth factor (CTGF) and type III collagen (Col III) in the renal interstitium. In addition, the same author also found that asiaticoside can improve myocardial fibrosis after myocardial infarction in rats. Zhao Sixia et al. [22] established an immune liver fibrosis model rat to investigate the effect of asiaticoside on the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (asiaticoside T) in the serum and hydroxyproline (Hyp) in the liver tissue of the model rat. The results showed that asiaticoside can reduce the degree of fibrosis in the liver tissue of the rat and significantly reduced the ALT and asiaticoside T levels in the serum and the Hyp level in the liver tissue of rats, indicating that asiaticoside has a beneficial effect on immune-mediated liver fibrosis. Ji et al. [23] found that asiaticoside can improve bleomycin (BLM)-induced lung fibrosis in rats, and that the mechanism of action may be related to the regulation of the TGF-β1/Smad pathway.
Another study found that asiaticoside can significantly reduce the degree of fibrosis in the lung tissue of mice with pulmonary fibrosis model. and the mechanism of action may be to improve pulmonary fibrosis by activating the A2AR-assisted cyclic adenosine monophosphate (cAMP)/R asperulosid-associated protein 1 (RAP1) signaling pathway [24]. Zhang et al. [25] induced pulmonary fibrosis in wild-type (WT) mice and A2AR knockout (A2AR-/-) mice using BLM, and then studied the intervention effect of asiaticoside. The results showed that the expression levels of bone morphogenetic protein 7 (BMP7) and p-Smad1/5 in the lung tissue of both WT mice and A2AR-/- mice were significantly elevated, suggesting that asiaticoside can reduce the degree of lung fibrosis by upregulating the BMP7/p-Smad1/5 signaling pathway through A2AR. Thus, asiaticoside can reduce the degree of fibrosis in various tissues and organs such as the lung, liver, kidney and heart. Its mechanism of action is related to the down-regulation of TGF-β1, CTGF, ALT, asiaticoside T and Hyp expression.
3 Anti-inflammatory effect and mechanism of asiaticoside
The NF-κB signaling pathway is closely related to inflammatory responses and plays an important role in regulating cellular inflammatory responses. When cells are stimulated, NF-κB can be activated, which in turn causes the overexpression of inflammatory factors downstream of the NF-κB signaling pathway, such as TNF- α, IL- 1β, IL-2, IL-6, nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) [26]. Related studies have found that On the one hand, asiaticoside can exert an anti-inflammatory effect by inhibiting the NF-κB signaling pathway and downregulating the expression of nitric oxide (NO), TNF-α, and IL-6 [27–28]. On the other hand, asiaticoside can also exert an anti-inflammatory effect by regulating the heme oxygenase 1 (HO-1) signaling pathway, inhibiting the production of pro-inflammatory factors and the activity of peroxidase[29].
As such, it can reduce the inflammatory response caused by hypoxic conditions by inhibiting the NF-κB/p38 signaling pathway [30], and reduce the inflammatory response caused by hyperoxic conditions by downregulating the expression of microRNA-155 (miR-155) and upregulating the expression of signal-transducing and inhibiting protein 1 (SOCS1) [31]. The protective effect of asiaticoside on β-amyloid 1-42 (Aβ1-42)-induced endothelial cell damage may be achieved by inhibiting the release of IL-1, IL-6, and TNF-α from endothelial cells [32]. It has also been reported that asiaticoside can inhibit mast cell-mediated allergic inflammation, and the mechanism may be related to the IgE high-affinity receptor (FcεRI) pathway [33]. Wu et al. [34] used rain frog toxin to replicate a mouse model of mild acute pancreatitis to study the intervention effect of asiaticoside. The results showed that asiaticoside can exert an anti-inflammatory effect by inhibiting the TLR4 pathway and reducing the expression levels of reactive protein kinase 3 (RIP3) and phosphorylated mixed lineage kinase domain-containing protein (p-MLKL) in pancreatic tissue. Thus, asiaticoside can improve various inflammatory responses. Its mechanism of action is related to downregulating the expression of IL-6, IL-1β, and TNF-α in the NF-κB pathway and inhibiting oxidative stress.
4. Anti-Alzheimer's disease effect and mechanism of asiaticoside
Alzheimer's disease (AD) is a common neurodegenerative disease characterized by progressive cognitive dysfunction. The pathogenesis of AD has been found to be related to inflammatory response, abnormal deposition of Aβ, and apoptosis [35].
According to literature reports, asiaticoside can relieve neuroinflammation by reducing the level of NO and the expression of iNOS mRNA in astrocytes [36]. Cai Pengfei [37] found that asiaticoside can improve the damage caused by Aβ to human umbilical vein endothelial cells (HUVEC), and its mechanism of action may be related to the inhibition of apoptosis and the expression of IL-1, IL-6, and TNF-α. Song et al. [38] studied the intervention effect of asiaticoside on Aβ1-42-induced apoptosis of human brain microvascular endothelial cells (hBMECs). The results showed that asiaticoside can inhibit hBMECs apoptosis, and the mechanism of action is related to the inhibition of the TLR4/NF-κB signaling pathway, the expression of TLR4, myeloid differentiation factor 88 (MyD88), tumor necrosis factor receptor-associated factor 6 (TRAF6), p-NF-κB p65 protein, and the nuclear translocation of NF-κB, as well as the expression of TNF-α and IL-6.
Centella asiatica extract can regulate the production of Aβ before neuropathy, thereby preventing the occurrence of AD [39]. Centella asiatica extract has a protective effect on HUVEC damage caused by Aβ1-42, and its mechanism of action may be related to up-regulating Bcl-2 expression and down-regulating Bax expression [40]. Xu et al. [41] found that aspertilloside has a significant reversing effect on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced AD, and its mechanism of action is related to the increase in the Bcl-2/Bax ratio. Aspertilloside also has strong blood-brain barrier penetration and has the potential to treat neurodegenerative diseases [42]. Thus, asiaticoside can improve AD, and its mechanism of action is related to the inhibition of HU-VEC apoptosis, the activity of the TLR4/NF-κB signaling pathway, and the expression of the inflammatory factors IL-1, IL-6, and TNF-α.

5. The effect and mechanism of asiaticoside in improving learning and memory
Centella asiatica extract can improve learning and memory in mice, and its mechanism of action may be related to reducing the deposition of Aβ in hippocampal tissue and upregulating the expression of synaptophysin (SYN) protein [43-44]; it may also be related to upregulating the expression of peroxisome proliferator-activated γ receptor (PPARγ) protein and downregulating the expression of inflammatory factors IL-6, TNF-α, and IL-1 [45]. Related studies have found that asiaticoside also has a good preventive effect on cognitive dysfunction in diabetes, and its mechanism of action may be related to the regulation of oxidative stress and the PI3K/Akt/NF-κB pathway [46].
6. Anti-depressant effect and mechanism of asiaticoside
Wang et al. [47] used the chronic mild stress (CMS) model mice to study the anti-depressant effect of asiaticoside. The results showed that asiaticoside can exert an anti-depressant effect by regulating the cAMP/protein kinase A (PKA) signaling pathway. Another study found that asiaticoside may exert an antidepressant effect by activating the brain-derived neurotrophic factor (BDNF) signaling pathway [48]. Hou et al. [49] compared the antidepressant effects of asiaticoside and an asiaticoside-borneol formula (FAB), and found that both have a certain antidepressant effect, with FAB being more effective than asiaticoside. FAB also has a more significant effect on upregulating BDNF and serotonin (5-HT) expression.
7 The effect and mechanism of asiaticoside in inhibiting scar hyperplasia and repairing skin damage
Studies have found that asiaticoside has unique advantages in inhibiting scar proliferation and repairing skin damage: on the one hand, asiaticoside can enhance cell migration activity and increase the wound healing rate [50-51]; on the other hand, asiaticoside can inhibit scar fibroblast proliferation and collagen synthesis, inhibit scar proliferation [52]. In vitro experiments have shown that the mechanism by which asiaticoside inhibits the proliferation of scar fibroblasts may be related to the inhibition of the expression of proteins involved in the RhoA/Rho kinase Ⅰ (RhoA/ROCK Ⅰ) signaling pathway [53], and may also be related to the down-regulation of the expression of type I and III collagen and TGF-β1 mRNA [54]. Related studies have found that in a rabbit ear scar model, as the administration time increases, the scar area gradually decreases, and the mechanism of action may be related to the reduced expression of TGF-β1 [55]. On this basis, Huang et al. [56] also found that asiaticoside can inhibit the formation of proliferative scars in rabbits by downregulating the expression of collagen I and III and IL-1β, IL-6, and IL-8 mRNA, as well as upregulating the expression of Smad7 and PPARγ mRNA, and this is dose-dependent. In another study, it was found that the reason for the inhibition of scar formation by asiaticoside may be related to the upregulation of heat shock protein 47 (HSP47) expression [57].
Wu Yanwen [58] established a deep second-degree burn rat model and intervened with asiaticoside. The results showed that the expression levels of cyclin and proliferating cell nuclear antigen (PCNA) in the skin tissue of the rat wound were elevated. At the same time, asiaticoside can inhibit excessive inflammatory response by inhibiting the nuclear translocation of NF-κB p65 protein, thereby promoting wound healing and shorten the healing time. Zhu et al. [59] found that coaxial nanofibers loaded with asiaticoside can promote the healing of deep second-degree burn wounds in rats by upregulating the expression of VEGF, PCNA and endothelial cell adhesion molecules (CD31) and downregulating the expression of TNF-α and IL-6. In addition, the role of asiaticoside at different stages of wound repair is different: it can promote scar proliferation during the proliferative phase of wound repair, but inhibit scar formation during the plastic phase of wound repair [60]. Another study found that asiaticoside can also promote the production of monocyte chemotactic protein 1 (MCP-1) to promote wound healing [61].
Nie et al. [62] found that the asiaticoside complex can reduce the inflammatory response in the wound of rats with diabetic skin ulcers (DCU) model, inhibit bacterial growth in the wound, promote wound healing, and upregulate the expression of VEGF, iNOS, endothelial nitric oxide synthase (eNOS) and CD34. The mechanism of action is related to downregulating the Wnt/β-catenin signaling pathway. Free skin flaps are a common means of skin burn and surgical repair. A skin flap is selected according to the skin defect, an area similar to the damaged skin is selected, and the flap is then transferred to the defect area for repair to achieve the purpose of repairing the skin defect [63]. A study found that asiaticoside can increase the survival area of skin flaps in rats, and the mechanism of action may be related to up-regulating the expression of superoxide dismutase (SOD) and VEGF and down-regulating the expression of inflammatory factors [64]. It has also been found that Centella asiatica-sodium alginate repair patches [65] and Centella asiatica nanoemulsions [66] prepared from asiaticoside have a good repair effect on skin damage.
This shows that asiaticoside can inhibit scar hyperplasia and repair skin damage. Its mechanism of action is related to inhibiting the expression of collagen types I and III and TNF-α, IL-6, and IL-1β, as well as the activity of the Wnt/β-catenin signaling pathway, and upregulating the expression of TGF-β1, VEGF, iNOS, and MCP-1.
8 The ameliorative effect and mechanism of asiaticoside on liver, lung and kidney damage
Studies have shown that asiaticoside has an ameliorative effect on both sepsis-induced lung damage and acute renal injury in sepsis. On the one hand, asiaticoside can inhibit the activity of the MAPK and NF-κB signal pathways by upregulating PPAR-γ expression, thereby ameliorating lung damage [67]. On the other hand, centella asiatica saponin can improve kidney damage by down-regulating the expression of IL-6 in the serum and the expression of iNOS protein in the renal tissue [68]. Zhang et al. [69] used a lipopolysaccharide/D-galactosamine-induced liver damage model mouse and intervened with centella asiatica saponin. The results showed that ASG can improve liver injury by inhibiting the expression of TNF-α and MAPK. In addition, Wang et al. [70] found that ASG can improve renal injury in rats with kidney disease, and the mechanism may be related to upregulating the mRNA expression of synaptophysin, endorphins, and podocin and downregulating the mRNA expression of junctional protein.
Dang et al. [71] studied the effect of asiaticoside on improving lung injury caused by hyperoxia through in vitro and in vivo experiments. The results showed that asiaticoside can reduce the levels of myeloperoxidase (MPO) and malondialdehyde (MDA) in the serum of rats with lung injury models, as well as the levels of TNF-α, IL-1β and IL-6, and increase the total antioxidant capacity (TAOC) level, to exert its effect of improving lung damage in rats. Further in vitro experiments using alveolar type II (AEC II) cells isolated from rat lung tissue showed that asiaticoside can significantly inhibit AEC II cell apoptosis. The mechanism of action may be related to upregulating the expression of nuclear factor E2-related factor 2 (Nrf2) and HO-1. Thus, asiaticoside can improve liver, lung and kidney damage, and its mechanism of action is related to inhibiting oxidative stress and inflammation and improving antioxidant capacity.
9 Other
Related studies have found that asiaticoside can be used to prevent radiation-induced DNA damage: after radiation treatment, mice treated with asiaticoside had increased survival rates and serum TAOC levels, TGF-β1 levels were reduced, and the mechanism of action may be related to enhanced antioxidant capacity [72]. Luo et al. [73] investigated the intervention effect of asiaticoside on rats with a spinal cord injury (SCI) model. The results showed that the levels of iNOS, TNF-α, IL-1β, IL-6, and NF-κB p65 in rat serum were reduced, and the levels of MDA, SOD, reduced glutathione (GSH) and glutathione peroxidase (GSH-Px) levels were increased. Further studies found that the expression level of p38MAPK in rat spinal cord tissue was significantly reduced, indicating that asiaticoside can improve spinal cord injury in rats through antioxidant, anti-inflammatory and inhibition of the p38MAPK signaling pathway. Fan et al. [74] found that ascorboside can restore motor function in rats with SCI and improve spinal cord neuron damage. The mechanism of action is related to the reduced expression of TNF-α and c-ascorboside p-ascorboside e-3 protein in spinal cord tissue. Eka et al. [75] investigated the hypoglycemic effect of ascorboside on diabetic mice. The results showed that After intervention with asiaticoside, the fasting blood glucose and glycosylated hemoglobin levels of diabetic model mice were reduced, and the insulin level was increased, suggesting that asiaticoside may have a good therapeutic effect on diabetes.

10 Conclusion
In recent years, with the deepening of research on asiaticoside by scholars at home and abroad, the main pharmacological effects and mechanisms of asiaticoside have been initially clarified: asiaticoside can exert anti-tumor effects by inhibiting tumor cell proliferation and inducing tumor cell apoptosis; it can exert anti-fibrosis effects by down-regulating the levels of TGF-β1, CTGF, ALT, asiaticoside T, and Hyp levels, it exerts an anti-fibrosis effect; it exerts an anti-inflammatory effect by down-regulating the expression of IL-6, IL-1β, and TNF-α in the NF-κB signaling pathway and inhibiting oxidative stress; it exerts an anti-AD effect by inhibiting vascular endothelial cell apoptosis, the activity of the TLR4/NF-κB signaling pathway, and the expression of the inflammatory factors IL-1, IL-6, and TNF-α; it exerts an anti-AD effect by inhibiting the expression of types I and III collagen and TNF-α, IL-6, IL-1β expression and Wnt/β-catenin signal pathway activity, upregulate TGF-β1, VEGF, iNOS, MCP-1 expression, and play a role in inhibiting scar hyperplasia and repairing skin damage; by mediating the MAPK/NF-κB signal pathway, inhibit MDA, iNOS, TNF-α, IL-6 expression and improved antioxidant capacity, exerting a role in improving liver, kidney and lung damage.
At present, most of the research on asiaticoside's anti-tumor effects focuses on the molecular mechanisms of cells in vitro, while pharmacological studies based on in vivo experiments are lacking. Therefore, subsequent anti-tumor research can focus on animal models. In addition, the research on asiaticoside's anti-inflammatory and anti-fibrosis effects most of the research focuses on the expression of related proteins, lacking research on the mechanism of action of specific signal pathways. Therefore, subsequent research can be conducted on the pharmacological effects and mechanisms of Centella asiatica extract from the perspective of signal pathways.
References
[ 1 ] National Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China: Part I [S]. 2020 Edition. Beijing: China Medical Science and Technology Press, 2020: 296.
[2] Weng Xiaoxiang, Huang Wenwu, Kong Deyun. Research progress on the triterpenoid components and pharmacological activities of Centella asiatica [J]. Chinese Journal of Pharmaceutical Industry, 2011, 42 (9): 709-716.
[3] Li Yanan, Li Zhihui, Huo Lini, et al. Research on the chemical composition of Centella asiatica[J]. Guangxi Traditional Chinese Medicine, 2015, 38(2): 78-80.
[ 4 ] DING Y, ZHANG Z, WANG S. Research progress of asiaticoside[J]. Shizhen National Medicine, 2016, 27(3): 697-699.
[ 5 ] ZHOU X, KE C L, LV Y, et al. Asiaticoside suppresses cell proliferation by inhibiting the NF-κB signaling pathway in colorectal cancer [J]. Int J Mol Med, 2020, 46(4):
1525-1537.
[6] HE Y G, PENG X H, ZHENG L, et al. Asiaticoside inhibits epithelial-mesenchymal transition and stem cell-like properties of pancreatic cancer PANC-1 cells by blocking the activation of p65 and p38MAPK[J]. J Gastrointest Oncol, 2021, 12(1): 196-206.
[7] Zeng Manhong, Chen Lingfeng. Study on the effect of asiaticoside on human gastric cancer cell line SGC-7901 [J]. Journal of Jiangxi College of Traditional Chinese Medicine, 2011, 23(6): 57-59.
[8] Liu Chenxu, Qin Sida, Xu Tao, et al. Effects of asiaticoside on apoptosis and VEGF and bFGF protein expression levels in breast cancer MCF-7 cells [J]. Advances in Modern Biomedicine, 2018, 18(7): 1314-1317.
[9] Wu Qian, Jiang Bo. The effect of asiaticoside on apoptosis of human osteosarcoma Saos-2 cells [J]. Modern Medicine and Clinical, 2019, 34(8): 2262-2267.
[10] MA Y, WEN J, WANG J, et al. Asiaticoside antagonizes proliferation and chemotherapeutic drug resistance in hepatocellular carcinoma (HCC) cells [J]. Med Sci Monitor, 2020, 26: e924435.
[11] Yang Lianjun. Bcl-2, Bax and tumor cell apoptosis [J]. Chinese Journal of Cancer Biotherapy, 2003, 3 (3): 232-234.
[12] Pan Jinli. Study on the mechanism of asiaticoside inducing apoptosis of human ovarian cancer cells A2780 [J]. Journal of Traditional Chinese Medicine, 2018, 33(4): 521-524.
[13] AL-SAEEDI F J. Study of the cytotoxicity of asiaticoside on rats and tumour cells[J]. BMC Cancer, 2014, 14(1): 220.
[14] AL-SAEEDI F J, BITAR M, PARIYANI S. Effect of asiaticoside on 99mTc-tetrofosmin and 99mTc- sestamibi uptake in MCF- 7 cells [J]. J Nucl Med Technol, 2011, 39 (4): 279-283.
[15] Li Y C, WANG H H, ZHANG R, et al. Antitumor activity of asiaticoside against multiple myeloma drug-resistant cancer cells is mediated by autophagy induction, activation of effector caspases, and inhibition of cell migration, invasion, and STAT-3 signaling pathway [J]. Med Sci Monit, 2019, 25: 1355-1361.
[16] Qi Luyu. Effects of asiaticoside and madecassoside on human embryonic fibroblasts in vitro [D]. Taiyuan: Shanxi Medical University, 2012.
[17] Qian Xingjia. Experimental study on the intervention effect of matrine and asiaticoside on human lung fibroblasts [D]. Nanjing: Nanjing University of Traditional Chinese Medicine, 2012.
[18] Ye Wenjing, Zhu Xiaochun, Wang Xiaobing, et al. Asiaticoside attenuates平阳霉素induced pulmonary mesenchymal fibrosis by inhibiting inflammation and fibrosis[J]. Chinese Journal of Pharmacology and Toxicology, 2016, 30 (1): 29-37.
[19] Zhu Dewei, Shen Yunhui. Protective effect of asiaticoside on mycophenolate mofetil-induced pulmonary fibrosis in mice [J]. Journal of Shanghai University of Traditional Chinese Medicine, 2020, 34 (6): 41-46.
[20] Wang Ce. Study on the effect of asiaticoside on renal fibrosis in rats with unilateral ureteral obstruction [J]. Chinese Medicine Clinical Research, 2017, 9(20): 1-4.
[21] Wang Ce. Experimental study on the effect of asiaticoside on myocardial fibrosis in rats with myocardial infarction [J]. Chinese Journal of Biochemical Drugs, 2014, 34(8): 19-22.
[22] Zhao Sixia, Zhang Ruzong, Yang Subei. Study on the effect of asiaticoside on immune liver fibrosis in rats [J]. Chinese Journal of Modern Applied Pharmacy, 2017, 34(5): 666-670.
[23] Ji Qijian, Xu Tie. Protective effect of asiaticoside on bleomycin-induced pulmonary fibrosis in rats [J]. Modern Medicine, 2014, 42 (11): 1304-1309.
[24] LUO J, ZHANG T, ZHU C W, et al. Asiaticoside might attenuate bleomycin-induced pulmonary fibrosis by activating cAMP and RAP1 signalling pathway assisted by A2AR [J]. J Cell Mol Med, 2020, 24(14): 8248-8261.
[25] ZHANG T, DAI J Y, YE W J, et al. Asiaticoside attenuates bleomycin-induced pulmonary fibrosis in A2AR-/- mice by promoting the BMP7/Smad1/5 signaling pathway [J]. Biochem Biophys Res Commun, 2020, 527(3): 662-667.
[26] Guo Limin, Lv Jieli, Zhang Laibin. Research progress on the anti-inflammatory mechanism of natural sesquiterpenoids [J]. Chinese Journal of Traditional Chinese Medicine, 2018, 43(20): 3989-3999.
[27] Linghu Lang, Jia Youjing, Chen Jing, et al. The effect of asiaticoside on lipopolysaccharide-induced inflammatory damage in astrocytes [J]. Journal of Zunyi Medical College, 2018, 41(2): 160-164.
[28] Jiang Chengcheng, Zhao Hengguang, Wu Yamei. Effects of asiaticoside on lipopolysaccharide-induced activation of nuclear transcription factor κB and inflammatory response in RAW264.7 cells [J]. Chinese Journal of Respiratory and Critical Care Medicine, 2010, 9 (4): 422-425.
[29] WAN J Y, GONG X, JIANG R, et al. Antipyretic and anti-inflammatory effects of Asiaticoside in lipopolysaccharide-treated rat through up-regulation of heme oxygenase-1 [J]. Phytother Res, 2013, 27(8): 1136-1142.
[30] Zhang T, Cai HJ, Yang L, et al. Asperulosid reduces hypoxic pulmonary hypertension in mice by inhibiting the NF-κB/p38 pathway. Chinese Journal of Pathological Physiology, 2019, 35(9): 1600-1607.
[31] MAI L J, FU X X, HE G, et al. Effect of asiaticoside on hyperoxia-induced bronchopulmonary dysplasia in neonatal rats and related mechanism [J]. Zhongguo Dang Dai Er Ke Za Zhi, 2020, 22(1): 71-76.
[32] ZHANG Z, CHEN L J, SUN Y H, et al. Effects of asiaticoside on the expression of inflammatory factors in endothelial cells induced by Aβ1-42 [J]. Chinese Journal of Biochemical Drugs, 2015, 35(1): 46-48.
[33] JIANG J Z, YE J, JIN G Y, et al. Asiaticoside mitigates the allergic inflammation by abrogating the degranulation of mast cells [J]. J Agr Food Chem, 2017, 65(37): 8128- 8135.
[34] WU K Y, YAO G H, SHI X L, et al. Asiaticoside ameliorates acinar cell necrosis in acute pancreatitis via Toll-like receptor 4 pathway [J]. Mol Immunol, 2020, 130: 122-132.
[35] Liu Chang, Meng Xianyong, Dong Xiaohua. The pathogenesis of Alzheimer's disease and research progress of therapeutic drugs [J]. Journal of Neuropharmacology, 2020, 10(4): 36-40.
[36] Mo Jucai, Ying Na, Xu Changliang, et al. Effects of asiaticoside on the release of inflammatory mediators from Aβ-induced astrocytes [J]. Zhejiang Traditional Chinese Medicine Journal, 2012, 47(11): 835-837.
[37] CAI P. Effects and mechanism of asiaticoside on Aβ1-42-induced endothelial cell damage [D]. Luzhou: Luzhou Medical College, 2014.
[38] SONG D, JIANG X, LIU Y, et al. Asiaticoside attenuates cell growth inhibition and apoptosis induced by Aβ1- 42 via inhibiting the TLR4/NF- κB signaling pathway in human brain microv ascular endothelial cells [J]. Front Pharmacol, 2018, 9: 28.
[39] HOSSAIN S, HASHIMOTO M, KATAKURA M, et al. Medicinal value of asiaticoside for Alzheimer's disease as assessed using single-molecule-detection fluorescence correlation spectroscopy, laser-scanning microscopy, transmission electron microscopy, and in silico docking [J]. BMC Complement Altern Med, 2015, 15(1): 118.
[40] ZHANG Z, CAI P, ZHOU J, et al. Effects of Asiaticoside on human umbilical vein endothelial cell apoptosis induced by Aβ1-42 [J]. Int J Clin Exp Med, 2015, 8(9): 15828-15833.
[41] XU C L, WANG Q Z, SUN LM, et al. Asiaticoside: attenuation of neurotoxicity induced by MPTP in a rat model of parkinsonism via maintaining redox balance and up-regulating the ratio of Bcl-2/Bax [J]. Pharmacol Biochem Behav, 2012, 100(3): 413-418.
[42] HANAPI N A, MOHAMAD A S, ABDULLAH J M, et al. Blood-brain barrier permeability of Asiaticoside, madecassoside and Asiatic acid in porcine brain endothelial cell model[J]. J Pharm Sci, 2020, 110(2): 698-706.
[43] Ying N, Yin ZJ, Yu H, et al. Intervention effect of asiaticoside on cognitive function in mice with diabetes model [J]. Zhejiang Journal of Integrated Traditional and Western Medicine, 2014, 24(3): 203-206, 184.
[44] Wang Xiaojing, Wang Dongxing, Xu Chunjing, et al. Therapeutic effect of asiaticoside on Alzheimer's disease [J]. Chinese Journal of Pharmaceutical Sciences, 2016, 6(13): 37-39, 92.
[45] HUANG Y L. Correlation between PPARγ and expression of inflammatory factors after intraperitoneal injection of Aβ1-42 in rats and intervention study of asiaticoside [D]. Luzhou: Southwest Medical University, 2017.
[46] YIN Z J, YU H Y, CHEN S, et al. Asiaticoside attenuates diabetes-induced cognition deficits by regulating PI3K/Akt/NF-κB pathway [J]. Behav Brain Res, 2015, 292: 288-299.
[47] WANG L Q, GUO T, GUO Y F, et al. Asiaticoside produces an antidepressant-like effect in a chronic unpredictable mild stress model of depression in mice, involving reversal of inflammation and the PKA/pCREB/BDNF signaling pathway [J]. Mol Med Rep, 2020, 22(3): 2364-2372.
[48] LUO L, LIU X L, MU R H, et al. Hippocampal BDNF signaling restored with chronic Asiaticoside treatment in depression-like mice [J]. Brain Res Bull, 2015, 114: 62-69.
[49] HOU T H, LI X B, PENG C S. Borneol enhances the anti-depressant effects of Asiaticoside by promoting its distribution into the brain [J]. Neurosci Lett, 2017, 646: 56-61.
[50] WARINTORN R, CHIRANAN K, et al. Depigmented centella asiatica extraction by pretreated with supercritical carbon dioxide fluid for wound healing application [J]. Processes, 2020, 8(3): 277.
[51] LEE J H, KIM H L, LEE M H, et al. Asiaticoside enhances normal human skin cell migration, attachment and growth in vitro wound healing model [J]. Phytomedicine, 2012, 19(13): 1223-1227.
[52] Ouyang Danwei, Shao Yan, Kong Deyun, et al. Inhibitory effect of asiaticoside and its chemical constituents on proliferation of scar fibroblasts [J]. World Clinical Drugs, 2014, 35(4): 215-220.
[53] Dai Libing, Pan Shu, Shen Yan, et al. Effects of asiaticoside on connective tissue growth factor and RhoA/ROCK-I regulatory signals in proliferative scar fibroblasts [J]. Chinese Journal of Pharmacy, 2010, 45 (14): 1067-1072.
[54] Li Shanshan. Effects of asiaticoside on proliferation of scar fibroblasts and expression of collagen and TGF-β1 mRNA [D]. Wuhan: Hubei University of Traditional Chinese Medicine, 2011.
[55] Zhou Z, Xiong W, Huang X, et al. Effects of asiaticoside on collagen fibers and TGF-β1 expression in proliferative scar tissue of rabbit ears [J]. Chinese Journal of Aesthetic Medicine, 2015, 24(21): 32-37.
[56] HUANG J, ZHOU X B, XIA L L, et al. Inhibition of hypertrophic scar formation with oral Asiaticoside treatment in a rabbit ear scar model [J]. Int Wound J, 2021 [2021-06-12].
[57] HU R, JIANG R. Effects of asiaticoside on wound healing and expression of heat shock protein 47 in scar formation in rats with burns [J]. Zhejiang Medical Journal, 2020, 42(1): 36-38, 101.
[58] WU Y. Research on the mechanism of asiaticoside in the treatment of burn wounds [D]. Nanjing: Nanjing University of Traditional Chinese Medicine, 2019.
[59] ZHU L F, LIU X Y, DU L, et al. Preparation of Asiaticosi- de-loaded coaxially electrospinning nanofibers and their effect on deep partial-thickness burn injury [J]. Biomed Pharmacother, 2016, 83: 33-40.
[60] Chen L, Wang T, Liu M, et al. Effects of asiaticoside on intervention at different stages of wound repair and immunohistochemical expression of transforming growth factor β1 and Smad3 in rabbit ear wounds [J]. Hebei Traditional Chinese Medicine, 2019, 41(10): 1532-1535, 1539.
[61] HOU Q, LI M, LU Y H, et al. Burn wound healing properties of Asiaticoside and madec Assoside [J]. Exp Ther Med, 2016, 12(3): 1269-1274.
[62] NIE X U, ZHANG H, SHI X J, et al. Centella asiatica iaticoside nitric oxide gel accelerates diabetic cutaneous ulcers healing by activating Wnt/β-catenin signaling pathway [J]. Int Immunopharmacol, 2020, 79: 106109.
[63] CHENG X F, WANG Z Y. Application of local skin flaps in the repair of facial skin defects [J]. World Latest Medical Information Digest, 2017, 17(11): 53.
[64] FENG X G, HUANG D, LIN D S, et al. Effects of Asiati- coside treatment on the survival of random skin flaps in rats [J]. J Invest Surg, 2021, 34(1): 107-117.
[65] Guo Jiaqi, Guo Minhui, Kong Songzhi, et al. Preparation of Centella asiatica glycoside-sodium alginate repair patch and its wound repair effect [J]. Chinese Herbal Medicine, 2020, 51(19): 4934-4942.
[66] PENG Q, LI J Y, LI H M, et al. Preparation and quality evaluation of asiaticoside nanomilks [J]. Chinese Journal of New Drugs, 2019, 28(10): 1276-1280.
[67] ZHANG LN, ZHENG JJ, ZHANG L, et al. Protective effects of Asiaticoside on septic lung injury in mice [J]. Exp Toxicol Pathol, 2011, 63(6): 519-525.
[68] ZHENG J J, ZHANG L N, WU M J, et al. Protective ef- fects of Asiaticoside on sepsis-induced acute kidney injury in mice [J]. Zhongguo Zhong Yao Za Zhi, 2010, 35(11): 1482-1485.
[69] ZHANG L, LI H Z, GONG X, et al. Protective effects of Asiaticoside on acute liver injury induced by lipopolysac- charide/D-galactosamine in mice [J]. Phytomedicine, 2010, 17(10): 811-819.
[70] WANG Z, LIU J T, SUN W S. Effects of Asiaticoside on levels of podocyte cytoskeletal proteins and renal slit diaphragm proteins in adriamycin-induced rat nephropathy [J]. Life Sci, 2013, 93(8): 352-358.
[71] DANG J W, LEI X P, LI Q P, et al. Asiaticoside attenuates hyperoxia-induced lung injury in vitro and in vivo [J]. Iran J Basic Med Sci, 2019, 22(7): 797-805.
[72] SHEN H Y, ZHU F, LI J S, et al. Protective effect of Asiaticoside on radiation-induced proliferation inhibition and DNA damage of fibroblasts and mice death [J]. Open Life Sci, 2020, 15(1): 145-151.
[73] LUO Y, FU C F, WANG Z Y, et al. Asiaticoside attenuates the effects of spinal cord injury through antioxidant and anti-inflammatory effects, and inhibition of the p38-MAPK mechanism [J]. Mol Med Rep, 2015, 12(6): 8294-8300.
[74] FAN L, LI X B, LIU T. Asiaticoside inhibits neuronal apoptosis and promotes functional recovery after spinal cord injury in rats [J]. J Mol Neurosci, 2020, 70(12): 1988-1996.
[75] EKA F, ELIN Y S, ELFAHMI E, et al. Antidiabetic activity of extract of fractions, and Asaticoside compound isolated from Centella Asiatica Linn. leaves in alloxan-induced diabetic mice [J]. Asian J Pharm Sci, 2017, 10(10): 268-272.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






