Natural Prebiotic Ingredient Oligofructose Supports Animal Nutrition Products Innovation
Fructooligosaccharides (FOS) are naturally occurring short-chain prebiotics with excellent stability and multiple physiological functions, widely used in food, beverages, health supplements, and animal feed. As a safe, residue-free, and antibiotic-resistant functional ingredient, FOS not only optimises gut microbiota composition but also enhances immunity, promotes mineral absorption, and improves lipid metabolism, making it an ideal alternative to antibiotics. Green Spring Technology provides 95% high-purity FOS powder that meets international food safety standards and is suitable for the formulation development of various health products.
FOS, as a high-quality feed additive, possesses excellent stability and high safety. It is non-toxic, harmless, leaves no residues in the body, and is not easily degraded by gastrointestinal enzymes, making it one of the ideal alternatives to antibiotics.FOS effectively optimises the structure of the intestinal microbiota in animals, promotes intestinal health, regulates protein and lipid metabolism, and enhances mineral absorption capacity. It possesses functional advantages similar to antibiotics but without the risks of residues or inducing antibiotic resistance, and is widely recognised as an efficient and safe prebiotic ingredient [1].
1. The outstanding physicochemical properties of oligofructose
1.1 Clear structure, natural source
Oligofructose, also known as oligofructose or sucrose trisaccharide oligosaccharide, has a molecular structure represented by G-F-Fn (G represents glucose, F represents fructose, and n = 1–3).It is a natural mixture formed by linking 1 to 3 fructose units via β-1,2 glycosidic bonds from sucrose, primarily including sucrose trisaccharide, sucrose tetrasaccharide, and sucrose pentasaccharide [2].
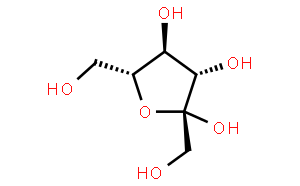
1.2 Refreshing sweetness, wide applicability
Oligofructose has a pure sweet taste and a refreshing mouthfeel. Its sweetness varies with purity: products with a purity of 55%–65% have a sweetness approximately 60% that of sucrose, while high-purity grades (96%) have a sweetness of only about 30% that of sucrose, making them more suitable for feed formulations where high sweetness is undesirable [3].
1.3 Appropriate viscosity, easy to process
Within the temperature range of 0–70°C, the viscosity of oligofructose is comparable to that of common corn high-fructose syrup and decreases gradually with increasing temperature, facilitating processing and mixing under various conditions [3].
1.4 Outstanding thermal stability and pH compatibility
Under neutral conditions (pH 7), oligofructose maintains excellent stability even at high temperatures of 120°C.In acidic conditions (pH 3), stability decreases at temperatures above 70°C, indicating that processing conditions should be appropriately controlled to maintain activity [3].
1.5 Slightly higher water activity than sucrose
Its water activity is similar to that of sucrose but slightly higher, which is beneficial for regulating the moisture state of material systems in certain applications [3].
1.6 Excellent comprehensive performance, suitable for various processes
Oligofructose has good solubility, heat resistance, starch aging resistance, non-colouring properties, formability, alkali resistance, and water retention capacity, with outstanding overall stability. The only point to note is its low hygroscopicity; moisture prevention is recommended during storage and production [3].
2 Physiological effects of oligofructose on animal nutrition
2.1 Maintaining intestinal microbiota balance
Oligofructose serves as a fermentation substrate for beneficial bacteria such as bifidobacteria, promoting their metabolism and proliferation, thereby inhibiting the growth of harmful bacteria and reducing the production of harmful metabolites. The increase in bifidobacteria is also associated with supporting immune function and nutrient synthesis [4].
2.2 Promoting Intestinal Peristalsis and Metabolism
Oligofructose promotes small intestine peristalsis, accelerates the expulsion of digestive tract contents, and improves faecal consistency. After fermentation, it produces organic acids that help regulate the intestinal acid-base environment, synergistically supporting the normal excretion of bodily waste with bifidobacteria, in a natural and safe process [4].
2.3 Supporting Immune Health
Oligofructose contributes to overall health primarily through the following mechanisms: first, it promotes the growth of beneficial bacteria, helping maintain intestinal microecological balance and stimulating the body's self-regulatory functions; second, it possesses auxiliary regulatory properties, supporting the body's defence mechanisms and participating in related physiological responses; third, it exhibits potential to regulate both humoral and cellular immunity. Oligofructose shows promising application prospects in maintaining health [4].
2.4 Promoting intestinal tissue health
Research indicates that adding oligofructose to animal diets promotes the proliferation of mucosal cells in the caecum and colon and supports mucosal structure. This effect may be related to the short-chain fatty acids produced when oligofructose is metabolised, which serve as an energy source for cells. Additionally, experiments have found that oligosaccharides can increase the length of intestinal microvilli in chicks, indicating a positive impact on intestinal structure.
3 Oligosaccharide Production Processes and Purification Technologies
3.1 Mainstream Production Processes
Industrial production primarily employs microbial fermentation. Enzyme preparations are produced through fermentation by specific moulds under suitable conditions, which then catalyse the conversion of sucrose with an efficiency exceeding 85%. This process has a high yield, a simple workflow, and product purity can reach 82%.
Fixed-cell technology enables continuous and automated production. Wet fungal cells are immobilised using sodium alginate to form gel beads, which are then loaded into a reactor for operation, resulting in high utilisation of the fungal cells.
Enzyme-based process optimisation involves introducing glucose oxidase to remove by-product inhibition, increasing product content from 50–60% in traditional methods to 71%.
3.2 Efficient purification methods
High-purity (≥95%) oligofructose powder can be obtained through chromatography or resin flow bed technology. The latter is a newly developed continuous purification production process in Japan and South Korea, with extremely high material utilisation rates.
Membrane filtration technology is also used for industrial purification, efficiently producing products with approximately 80% purity, which can be further processed to over 90%, offering good economic benefits.
4 Promising Prospects for Oligofructose in Animal Nutrition
Over the past half-century, antibiotics have made significant contributions to the development of the livestock industry. However, their side effects have gradually become apparent, such as increased risks of endogenous infections and secondary infections in animals, impaired immune function, and potential impacts on human health through the food chain. The emergence of oligosaccharide additives has provided the industry with new solutions.
Oligofructose itself does not directly act on the animal body. Its core mechanism lies in significantly promoting the proliferation of beneficial bacteria such as bifidobacteria, thereby increasing their proportion in the intestinal microbiota. While maintaining the overall bacterial balance, it effectively inhibits the colonisation and reproduction of harmful bacteria such as Salmonella and E. coli.
Studies have shown that adding oligosaccharides to feed can help improve daily weight gain and feed conversion efficiency in animals, enhance their disease resistance, and significantly reduce the number of pathogenic microorganisms in the digestive tract and livestock products. Currently, oligosaccharides are regarded as a class of functional feed additives with broad development potential [10].

As a green and safe new feed ingredient, oligosaccharides selectively promote the growth of beneficial bacteria, inhibit the colonisation of pathogenic bacteria, and achieve antibiotic-like health benefits while avoiding drug residues and antibiotic resistance issues. Additionally, their widespread availability and controllable costs have made them one of the most mature varieties among oligosaccharide additives. With ongoing research and the maturation of application technologies, oligofructose demonstrates significant advantages and broad prospects in promoting the sustainable and healthy development of the livestock industry [11].
Green Spring Technology's oligofructose raw materials comply with regulations in multiple countries, including the EU, the United States, and Japan, and are certified by BRC, IFS, ISO 9001, ISO 22000, HALAL, and KOSHER, enabling global market access.
We are committed to collaborating with global clients to provide customised raw material solutions and technical support, jointly driving the development of next-generation health products. Please get in touch with us at helen@greenspringbio.com to request samples and technical documentation.
References:
[1] Huang Haiqing, Wang Yizhen. Application and research progress of oligofructose in animal nutrition [J]. Feed Wide Angle, 2002 (16): 26-27.
[2] Xu Xilin, Lin Qingsheng, Lai Fengying. Production of fructooligosaccharides from sucrose fermentation [J]. Food Industry Science and Technology, 1997 (4): 34-37.
[3] Wang Shihua, Peng Limin, Zhang Hui, et al. Development and application of fructooligosaccharides [J]. China Dairy Industry, 2002 (2): 31-34.
[4] Tang Shengqiu, Dong Xiaoying, Zou Xiaoting. Oligofructose and its application in animal nutrition [J]. Additive World, 2003 (10): 40-42.
[5] Yang Yuange, Luo Faxing. Oligofructose - a new type of functional food base [J]. Sugarcane Sugar Industry, 2002 (3): 35-39.
[6] Wang Shihua, Bai Wenzhao, Luo Jinhua, et al. Application of oligofructose in feed [J]. Cereals, Oils and Feed Industry, 2002 (3): 25-26.
[7] Tang Jun, Zhang Haitao. Properties of oligofructose and new fermentation process for its production [J]. Chemical Industry and Engineering Technology, 1999, 20 (2): 11-12.
[8] Wang Duoren. Development of oligofructose [J]. Guangxi Sugarcane, 2002(4): 29-34.
[9] Li Xiuqin, Yang Xinghui, An Suxin. Purification method of oligofructose [J]. Hebei Chemical Industry, 2003(1): 4-7.
[10] Lin Qinlu, Qin Dan, Jin Yanghai. Production and application of functional factors of oligofructose [J]. Chinese Food and Nutrition, 2002 (4): 20-22.
[11] Yao Jianguo, Xia Dong, Li Ruzhi. A new type of feed additive, oligofructose [J]. Additive World, 2000 (5): 15-16.
-
Prev
What Is the Benefit of Fructooligosaccharides?
-
Next
Fructooligosaccharide (FOS) Ingredient Empowering Antibiotic-Free Feed Formulations


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese



