What Are Glutathione Uses in Hindi?
Glutathione (GSH) is a tripeptide containing sulfhydryl groups and γ-amide bonds composed of glutamic acid, glycine, and cysteine; it exists in two types: oxidized (GSSG) and reduced (GSH), and the oxidized form is converted to the reduced form containing reactive sulfhydryl groups by the action of the enzyme glutathione reductase in the cytoplasm of the human body. GSH is found in many plants, animals and microorganisms in nature and is a peptide synthesized naturally in the cells of various organisms and is involved in the maintenance of cellular biological functions.
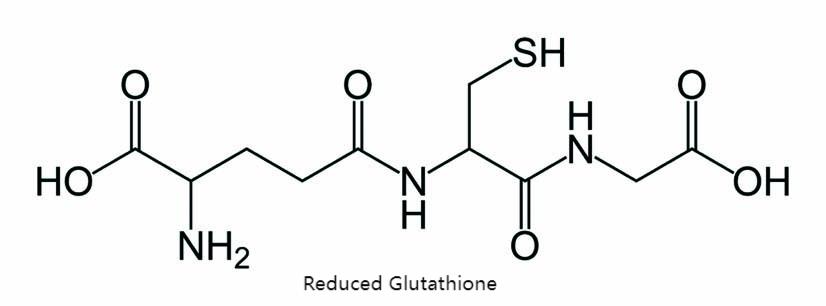
Glutathione is most abundantly found in animal liver, wheat germ and yeast [1], and also in human blood and fruits and vegetables such as tomatoes and pineapples. Because of its detoxification, immunity and antioxidant capacity, glutathione in Hindi is widely used in the detoxification of clinical heavy metals and fluoride or certain drugs and is added to various kinds of immunity-enhancing, anti-tumor and anti-aging functional foods, or as an ingredient in various drugs for hepatitis and hemolytic diseases.
1. Biological Use of Glutathione
GSH exerts its important physiological functions mainly through its γ-glutamine bond and reactive sulfhydryl groups on cysteine residues, which are involved in the metabolism of various substances such as glucose metabolism (tricarboxylic acid cycle), amino acids and lipids. The sulfhydryl group is the most important functional group, and its function is also the most important, as a substance that prevents the oxidation of proteins in the body and fights against oxygen radicals to participate in a variety of important redox reactions in the body and play an antioxidant and detoxification role; γ-glutamine bond prevents the cleavage of peptidases in the cell and the plasma membrane γ- glutamyltranspeptidase (γ-GT) and stabilizes the cell in the cell; and γ- glutamine bond prevents the cleavage of peptide enzymes and plasma membrane γ-glutaminyltranspeptidase. and stabilizes it in the cell [2].
1.1 Immunological Functions of Glutathione
Glutathione can increase the activity of enzymes related to immune response. Zhou Tingting et al. [3] added a certain amount of GSH to the feed of juvenile Jifu tilapia to study the effects of non-specific immune-related enzymes. The results showed that the addition of a certain amount of GSH to the feed of juvenile tilapia increased the activities of non-specific immune-related enzymes, such as lysozyme, alkaline phosphatase, and acid phosphatase in the serum and liver of juvenile tilapia as compared with that of control fish, and that the fish do not have specific immune system, and the stimulation of foreign substances mainly manifests phagocytosis and phagocytic response. Fish do not have a specific immune system, and the stimulation of foreign substances mainly manifests phagocytosis and the production of antimicrobial substances, so the increase in the activities of these enzymes implies that GSH can improve the non-specific immune ability of tilapia.
Glutathione can repair liver injury and improve immune function. Chang et al. [4] applied GSH to patients with hepatitis B and found that GSH could repair liver injury caused by alcohol, drugs and viral hepatitis, improve liver function and enhance immunity to a certain extent.
1.2 Antioxidant and Detoxification Effects of Glutathione
Glutathione is a major intracellular antioxidant in living organisms [5], which reduces cellular damage caused by reactive oxygen complexes by increasing the metabolism of electrophilic products. It is mainly detoxified by glutathione-S-transferase (GST) and glutathione peroxidase (GSHPX). Oxygen radicals (-OH) can be involved in signaling and immune responses, but because they can capture an electron from other substances, excessive oxygen radicals can lead to lipid peroxidation or inactivation of certain enzymes in the cell, resulting in cellular tissue damage [6].
GSHPX is found in the cytosol and mitochondria of eukaryotic cells, and its detoxification mechanism involves the reduction of H2O2 to H2O, thus breaking the -OH chain and protecting the cells from oxygen radicals. GSHPX is found in the cytosol and mitochondria of eukaryotic cells, and its detoxification mechanism is to reduce H2O2 to H2O, which breaks the -OH chain and protects the cells from the toxicity of oxygen radicals.
Peng Linxiu [7] et al. investigated the effects of glutathione in the treatment of renal injury induced by anti-tuberculosis drugs. The results showed that isoniazid and rifampicin-induced renal tissue inflammation was significantly reduced with the participation of glutathione. The mechanism of action of glutathione is that it can reduce the level of urea nitrogen (BUN) in the serum of rats, and exerts its antioxidant and detoxification effects through the regulation of metabolism.
1.3 Glutathione Is Involved in the Absorption of Substances
Glutathione can reduce Fe3+ to Fe2+ and increase the solubility of Fe in the body to promote the absorption of Fe. GSH is also involved in the uptake and absorption of amino acids, i.e., the γ-glutamyl cycle [8], which plays a role in the renal tubules, brain tissues, and intestinal tracts, and is catalyzed by the extracellular γ-glutamyltransferase, which combines the γ-glutamyl group of GSH with the extracellular amino acid and carries it into the intracellular release. It is catalyzed by extracellular γ-glutamyl transpeptidase, which binds the γ-glutamyl group of GSH to extramembrane amino acids and carries them to the intracellular compartment for release, while the glutamyl group is regenerated from free amino acids to be utilized as GSH; it has been demonstrated that proline cannot be absorbed and transported through the γ-glutamyl cycle.

1.4 Other Uses
Glutathione plays a major role in cellular regulation of metabolism and anti-injury, and it participates in the tricarboxylic acid cycle and accelerates the metabolism of amino acids, sugars and lipids. Zhang Jianhua [9] randomly divided 82 patients with alcoholic liver disease into a research group and a reference group, the research group was treated with reduced GSH and the reference group was treated with potassium chloride injection, and the results were observed and analyzed, and it was concluded that reduced GSH could effectively reduce the biochemical indexes of the patients, alleviate the symptoms, and have high safety, which is related to its participation in the biochemical metabolism in the body.
Reduced Glutathione has some neuroprotective effects on vascular dementia (VaD). Xiang et al.[10] divided mice into the sham operation group, VaD group, GSH50 group and GSH100 group, and examined their learning and memory abilities and the degree of hippocampal neuron damage respectively. The results showed that the learning and memory abilities of VaD mice were impaired, and the GSH100 mice were significantly improved after the administration of high dose GSH. Compared with the sham-operated group, the hippocampal neuron positive cells of VaD mice were significantly decreased. However, the positive cells of hippocampal neurons in VaD mice were significantly increased after continuous application of high doses of GSH.
2.What Are the Detection Methods of Glutathione?
Since the discovery of glutathione in 1888, through continuous human research, glutathione has been found to have important roles in various fields such as nutrition and biology, and therefore it is clinically significant to explore it in greater depth. Due to the influence of the form of glutathione, temperature, pH and other factors, the accurate detection of glutathione is still difficult and needs to be solved. Because of the differences in the samples, conditions and composition of glutathione, the methods of glutathione analysis are different.
2.1 Electrochemical Analysis
Electrochemical Methods (EM) is a method that detects the concentration of a substance by converting it into an electrical signal based on the chemical (e.g., electrode potential, amount of electricity, current, etc.) or physical (e.g., concentration, chemical composition, etc.) properties of a solution and the redox reaction that occurs at different potentials [11]. Wang, Wenlei et al. [12] used a combination of electrochemical detection and microfluidic chip electrophoresis to study GSH in a single human hepatocellular carcinoma cell, which could be detected on a gold/mercury electrode without other derivatization steps. Yang Peihui et al. [13] used cyclic voltammetry to study chromium (VI) and GSH and found that there was a linear relationship between the peak signal of chromium (VI) and the concentration of GSH, and established an indirect electrochemical method for the determination of GSH.
The electrochemical analysis method has high accuracy, strong sensitivity, wide measuring range, and simple and cheap equipment, but it is easy to be interfered with by other substances and has low selectivity.
2.2 Iodometric Method
Iodimetry is a method of determining the content of a substance by redox titration using iodine as the oxidizing agent and iodide as the reducing agent. Iodimetry makes use of the oxidizing property of potassium iodate to oxidize the reduced sulfhydryl groups in GSH. Excess potassium iodide reacts with potassium iodate, and the endpoint is determined by the change in color of the starch indicator to determine the content of GSH [14]. Liao Fei et al. used the UV-absorption iodometric method for the determination of reduced GSH and trace vitamin C[15].
The iodometric method is simple and fast, but it has low sensitivity, and poor specificity, and is easily interfered with by other substances such as proteins, so it is not commonly used in the actual determination of glutathione.
2.3 Fluorescence
The fluorescence method (FM) is a quantitative or qualitative method to analyze glutathione by detecting the fluorescence intensity emitted by the sample after absorbing ultraviolet light [16]. Cao Xinzhi et al. [17] used a fluorescence spectrophotometer for the determination of GSH in wheat embryo based on the principle that GSH binds to OPT (o-phthalaldehyde) in alkaline environment to form a stable complex, and that this complex emits blue fluorescence in the presence of UV light; and the reproducibility of this method was good. Based on the characteristic that reduced GSH can react with OPT and form a fluorescent system, Zhang Jing [18] et al. detected the content of reduced GSH in 10 marine organisms and concluded that the GSH content of seven marine organisms, including fish, shrimp and shellfish, was close to that in the blood of terrestrial animals, but was significantly higher than that in terrestrial plants.
The fluorescence method for glutathione detection is easy to operate, with high sensitivity and fast reaction speed, and the fluorescent substance has a certain degree of stability, but if there is no suitable separation method, the detection will be easily interfered by the outside world.
2.4 High Efficiency Capillary Electrophoresis (HECE)
High Performance Capillary Electrophoresis (HPEC) is a technique that uses a capillary as the separation channel and a high voltage electric field as the driving force to realize the separation of samples according to the different distribution behaviors and flow rates of each component [19]. Zhu Longbao [20] used 1/15 mmol/L phosphate buffer (pH=7.4) and a quartz capillary column as the separation channel to examine the content of GSH in brewer's yeast, and the lowest limit of detection (LOD) was 1.94 mg/L. Liu Tao [21] selected NaH2PO4- buffer with a concentration of 500×10-2 mol/L as the detection method for the determination of GSH in monocytes, and obtained the results of GSH in the extracts from the abdominal cavities of rats. The average GSH content per cell in the extract of rat abdominal mast cells was 187 fmol.
The high performance capillary electrophoresis (HPECE) method is easy to operate and requires that the GSH content in the sample should not be less than 50 μmol/L. However, the sensitivity is low and the sample processing is complicated and troublesome, so it is only suitable for the detection of small samples.
2.5 High Performance Liquid Chromatography (HPLC)
High Performance Liquid Chromatography (HPLC) uses a high-pressure infusion system to pump single solvents of different polarities, mixed solvents, etc., into a column and inject the samples to be measured. The solubility of each component of the samples varies, and the adsorption and desorption processes between the stationary phase of the column and the solvents result in a separation, which ultimately enters the detector for detection [22]. The solubility of each component of the sample is different. A modified high performance liquid chromatographic (HPLC) method was developed by Chen Liangli et al [23] to analyze GSH in yeast crude extracts. In the detection of reduced GSH, an effective separation from the heterogeneous peaks was achieved. Zhai et al. [24] used high performance liquid chromatography (HPLC) to analyze the total sulfhydryl groups (-SH) and reduced GSH in the seeds of Candelilla officinalis. The results showed that the average value of total sulfhydryl was 6.06 μmol/g, and that of reduced GSH was 4.0 μmol/g. The HPLC method is one of the most popular methods for the determination of total sulfhydryl and reduced GSH.
High performance liquid chromatography (HPLC) is the most direct and effective method for the determination of hydroxyl groups in complex biological samples in recent years, which has the advantages of accurate results, fast analytical speed, high selectivity, a wide range of application and high stability, with the disadvantages of cumbersome operation procedures, low sensitivity, time-consuming, and the requirement that the minimum glutathione content in the sample is 50 μmol/L. The results showed that the average values of total mercaptans and reduced GSH were 6.06 μmol/g, and 4.0 μmol/g of reduced GSH.
3.Conclusion
In recent years, with the in-depth study of glutathione, its functions have been better understood. Nowadays, glutathione has been widely used in clinical medicine, animal husbandry, food industry and other fields.
References:
[1] Yang Changyan, Ba Qingyun, Zhang Zhixin, et al. Clinical study on the treatment of anti-tuberculosis drug-induced hepatitis with bicyclic alcohol combined with reduced glutathione [J]. Modern Drugs and Clinics,2017,32(04):653-656.
[2] SONG Zengting, JIANG Ning, ZHANG Aizhong, et al. Progress of research on the biological functions of glutathione [J]. Feed Research,2008(09):25-27.
[3] ZHOU Tingting, CAO Junming, HUANG Yanhua, et al. Effects of dietary glutathione on growth, tissue biochemical indexes and non-specific immune-related enzymes in Jifu tilapia [J]. Journal of Aquatic Sciences,2013,37(05):742-750.
[4] Chang JG. Effects of reduced glutathione on liver fibrosis and immune function in patients with hepatitis B [J]. China Practical Medicine,2019,14(35):124-126.
[5] Kritzinger E C. Winemaking practices affecting glutathione concentrations in white wine [D]. Stellenbosch: Stellenbosch University,2012.
[6] Cheng YK. Detoxification of glutathione and its toxic metabolites [J]. Advances in Biochemistry and Biophysics,1994(05):395-399+472.
[7] PENG Linxiu, XIE Tong, SAN Jinjun. Metabolomics of renal injury caused by antituberculosis drugs and therapeutic effects of glutathione based on gas chromatography-mass spectrometry[J]. Analytical Chemistry,2020:1-11.
[8] ZHENG Yun-Lang. Biological functions of glutathione[J]. Biological Bulletin,1995(05): 22-24.
[9] Zhang JH. Analysis of the pharmacological effects of reduced glutathione and clinical observation [J]. Psychology Monthly ,2020,15(09):207.
[10] Xiang WJ, Zhou ZX, Jiang YL, et al. Neuroprotective effects of glutathione in mice with vascular dementia [J]. Chinese Journal of Geriatric Cardiovascular and Cerebrovascular Disease,2020,22(05): 529-533.
[11] YU Ji-Mei. Application of electrochemical analysis in food safety [J]. Jiangxi Chemical Industry, 2012(04):116-118.
[12] Wang Wenlei , Jin Wenrui . Determination of glutathione in single human hepatocellular carcinoma cells by microfluidic chip electrophoresis/electrochemistry [J]. Chromatography,2007(06):799-803.
[13] YANG Peihui, ZHAO Qiuxiang, CAI Jiye. Electrochemical Detection of Glutathione in the Presence of Chromium (VI) [J]. Chinese Journal of Biochemical Drugs,2004(05):273-275+296.
[14] Fan Chongdong , Wang Miao , Wei Gongyuan , et al. Progress in the determination of glutathione [J]. Biotechnology,2004(01):68-70.
[15] Liao Fei , Yang Xiao , Kang Gefei , et al. Determination of trace vitamin C and reduced glutathione by ultraviolet absorption iodometry [J]. Journal of Chongqing Medical University,2003(03):372-373.
[16] Chen GN , Sun H , Shan YL , et al. Rapid fluorescence detection of glutathione [J]. Journal of Clinical Investigation,2001(01):11-12.
[17] Cao Xinzhi , Chen Yan . Determination of glutathione in wheat embryo by fluorescence method [J]. Grain and Feed Industry,2001(11):46-47.
[18] Zhang J, Ji H W, Zhou J, et al. Determination of glutathione in marine organisms by fluorescence spectrophotometry [J]. Journal of Zhanjiang Ocean University,2005(04):32-34.
[19] ZHANG Xiu-ling. Application of high-performance capillary electrophoresis in drug analysis [J]. Tianjin Pharmacology,2004(01):56-60.
[20] ZHU Longbao, WEI Shenghua, GE Fei, et al. Determination of glutathione in brewer's yeast by high performance capillary electrophoresis [J]. Food Industry Science and Technology,2011,32(08):394-396.
[21] LIU Tao. Determination of glutathione in single cells by capillary electrophoresis with electrochemiluminescence [D]. Qingdao University of Science and Technology,2010.
[22] Zhong H, Zhang H, Xu H P . Advances in the determination of glutathione [J]. Amino Acids and Bioresources ,2014,36(01):23-26.
[23] CHEN Liangli, DANG Aiping, DU Weili, et al. Determination of glutathione in yeast cells by modified high performance liquid chromatography [J]. Journal of Three Gorges University,2018,40(04):99-102.
[24] ZHAI Fangyuan , ZHANG Xiaoyong , CUI Shengyun . Determination of reduced glutathione and total sulfhydryl groups in the seeds of Corylus aromaticus by high performance liquid chromatography [J]. Journal of Analytical Science ,2016,32(02):257-260.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






