The Foods That Contain Beta Carotene
Beta-carotene [Greek β and Latin carota (carrot)] is a natural coloring agent, dietary supplement and pro-vitamin A (VA) that is widely used in foods, feeds, supplements and cosmetics. It belongs to the carotenoid family. The global carotenoid market was worth 1.5 billion US dollars in 2014 and is expected to reach nearly 1.8 billion US dollars in 2019, with a compound annual growth rate (CAGR) of 3. 9% [1] , and the carotenoid with the largest market value is β-carotene (US$26.1 billion in 2010, expected to increase to US$33.4 billion in 2018, with a CAGR of 3.1%) [2] .
Carrot (Daucus carota L.), belonging to the Apiaceae family (formerly known as the parsnip family), is a direct sowing cool-season crop that produces the best color in its roots when the air temperature is 18–21 °C [3]. Carrots contain β-carotene, which comes in different colors, with purple, yellow, and orange being the most common. Roszkowska et al. [4] found that the β-carotene content of three different colored carrots, namely orange, purple, and white, was 74.2, 9.1, and 1. 8 mg/100 g. The content of carotenoids in carrots is an important indicator for evaluating the quality of carrot varieties and is the main basis for development and utilization [5]. The total carotenoid content of the edible part of carrots ranges from 6000 to 54800 μg/100 g[6] , and β-carotene accounts for 45% to 80% of the total in orange carrots[7] .

Different varieties of carrots contain different amounts of β-carotene due to various geographical and environmental factors [8]. Mendelová et al. studied nine different varieties of carrots and found that Kamaran F1 had the highest β-carotene content (213.66 mg/100 g). The β-carotene content of the same variety varies depending on the pre-processing and preservation methods [9]. China is the world's leading producer of carrots. Since the β-carotene content of carrots is the highest (47.5–1030 μg/g) among all vegetables [10], and they are cheap and easy to obtain, they can provide a source for extracting large amounts of natural β-carotene. This paper describes the structure and properties, uses, and recent research on the extraction of β-carotene from carrots at home and abroad, with the aim of providing a reference for the extraction of β-carotene.
1 Physicochemical properties of β-carotene
1.1 Properties of β-carotene
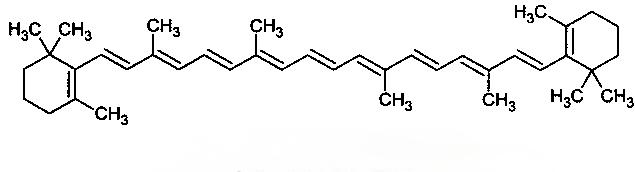
The chemical structural formula of β-carotene is shown in Figure 1. Molecular formula C40 H 56 , molecular weight 536.88 , with 4 isoprene centers in the middle and a violet ketone ring at the end, melting point 176~182 ℃ , sensitive to light, heat and oxygen. There is no asymmetric carbon atom in the all-trans molecule, and is not optically active. High temperatures and pressures (625 MPa, 117 °C) are detrimental to it and can easily cause isomerization [11]. Its isomers (see Figure 1) are mainly: 9-cis, 13-cis and 15-cis β-carotene. Due to the poor stability of β-carotene and the limitations of its solubility, it can be encapsulated in liposomes such as microcapsules, cyclodextrins, and vesicles. It can also be prepared into a liquid crystal system using surfactants, or it can be prepared into an emulsion to reduce losses during preparation and storage and to improve solubility and bioavailability[12]. Zhou Qingxin et al. [13] compared the effects of the antioxidants EDTA-2Na, L-ascorbic acid, vitamin E acetate, and their combinations on the stability of β-carotene microemulsions during processing and storage. The degradation value of β-carotene was measured by a colorimeter, and the results showed that EDTA-2Na can better stabilize β-carotene microemulsions.
1.2 β-Carotene uses
It has been reported that more than 700 types of carotenoids have been identified, of which about 50 types appear in the daily diet of humans [14]. Beta-carotene is found in various tissues of the human body, mainly stored in fat and the liver [15]. The human body cannot synthesize beta-carotene itself and must obtain it from food. Factors that affect the bioavailability and bioconversion of beta-carotene in the human body include: edible fats and oils, plant type, fiber, temperature, etc. Among them, edible fats and oils can promote the formation of micelles by beta-carotene, which is beneficial to human absorption. Fiber is not conducive to the release of beta-carotene from cells and has poor biological selectivity[16] .
Natural β-carotene is all-trans, and all-trans β-carotene has a higher bioavailability than its isomers [17]. The biological conversion rates of 9-cis and 13-cis are 38% and 53% respectively, while the all-trans is 100% [18]. Beta-carotene can be obtained from algae, fruits and vegetables, as well as some fungi. Currently, the source of beta-carotene on the foreign market is chemical synthesis, which accounts for 90%. Beta-carotene obtained from natural foods is more beneficial to human health[19] , and the amount of beta-carotene absorbed from plants ranges from 5% to 65%[20].
Beta-carotene has a strong antioxidant effect because it contains many unsaturated conjugated double bonds and consists only of the two elements carbon and hydrogen. It can scavenge singlet oxygen (1O2) and superoxide anion (O2-) radicals, with each molecule able to scavenge up to 1,000 radicals. It is an important source of vitamin A (VA). A lack of VA can lead to night blindness and xerophthalmia, while excessive intake of VA can cause teratogenicity, osteoporosis, liver damage and other adverse effects[21]. When the body ingests large amounts of β-carotene, it is converted by the enzyme β-carotene 15,15'-monooxygenase not only can it replenish VA in time, but it can also effectively reduce the incidence of certain diseases. For example, it has recently been discovered that beta-carotene can inhibit neuroblastoma, the most common extracranial solid tumor in childhood[22]. 9-cis-beta-carotene can prevent macrophages from transforming into foam cells and inhibit the process of atherosclerosis[23]. The oxidation products of beta-carotene: beta-ionone, 5,6-epoxy-beta-ionone and dihydrocapsaicin (DHA) can provide a source of flavor and fragrance [24]; beta-carotene also has anti-mutagenic, chemical preventive, photoprotective, enhancing intercellular communication and regulating the activity of the immune system. However, for heavy smokers and drinkers, large amounts of beta-carotene may increase their risk of lung cancer [25].

2 Extraction method of beta-carotene from carrots
Beta-carotene is located on the chloroplasts of cell tissue. To obtain beta-carotene from carrots, the cell wall needs to be destroyed. Common methods of cell disruption include mechanical grinding, physical ultrasonic methods, chemical dissolution and enzymatic disruption. The cell wall is harder than the cell membrane, which can be destroyed by osmosis, so the disruption is mainly concentrated on the cell wall. From a physical point of view, freezing carrots is more conducive to the extraction of β-carotene because the components between the cell walls of the carrots are destroyed during the thawing process, causing the cell walls to dissociate. Coupled with the formation of ice crystals, this further causes damage to the structural organization of the carrots, so that β-carotene is easily extracted [26]. Moreover, frozen carrots have less nutrient loss [27].
In recent years, domestic and foreign methods for extracting β-carotene from carrots have included: organic solvent method, ultrasonic-assisted extraction method, microwave-assisted extraction method, microemulsion method, accelerated solvent extraction method, enzymatic dissolution and extraction method, and supercritical fluid method.
2.1 Organic solvent method
Beta-carotene powder is fat-soluble and soluble in non-polar solvents such as ether, chloroform and oils, but hardly soluble in methanol and ethanol. The principle of organic solvent extraction is that under the action of diffusion and osmosis, solvent molecules enter the cells through the cell wall, dissolve the soluble substances, and the solvent continues to enter the cells through the concentration difference. Finally, when equilibrium is reached, the extract flows from the cells to the solvent, thus achieving the purpose of extraction [28]. Although β-carotene is insoluble in polar solvents such as methanol and ethanol, methanol and ethanol are used in the extraction of β-carotene from carrots in organic solvents. This is because fresh carrots contain a lot of water (86% to 89%), and the purpose of adding polar solvents is to mix with water to increase the permeability of the non-polar solvent and thereby facilitate the extraction of β-carotene [29]. Nowak et al. [30] used a mixed solvent of hexane and 96% ethanol (1:1 volume ratio) to extract β-carotene from 17 different varieties of carrots. The result was that the Kazan F1 variety of carrots contained (17.1±3.7) mg/100 g (fresh weight) of β-carotene.

Organic solvents are the most widely used and inexpensive, but they are also highly toxic and require large amounts to be used. Therefore, environmentally friendly solvents with low usage are needed to extract β-carotene [31]. Varón et al. [32] used modeling methods: Hansen solubility parameter (HSP) and COSMO-RS, to compare the green and low-toxicity extraction solvents: 2-methyl tetrahydrofuran (2-MeTHF), dimethyl carbonate (DMC), cyclopentyl methyl ether (CPME), isopropanol (IPA), ethyl acetate and hexane on the extraction of carotenoids from carrots. The results of the two simulations are as follows: the HSP model shows that non-polar or low-polar solvents are more conducive to the extraction of carotenoids, while the COSMO-RS model indicates that the content of carotenoids in CPME, 2-MeTHF and ethyl acetate is higher than in hexane.
Experimental verification of the results is closer to the results simulated by the COSMO-RS model, and the content of carotenoids in the CPME solvent is the highest (78. 4 mg/100 g, dry weight), of which β-carotene accounted for 66%. This means that these solvents with lower toxicity and biodegradability can replace hexane for the extraction of carotenoids from carrots, which is very important for the food industry. Rajabi et al. [33] established a COSMO-RS model and used ionic liquids as extractants to extracted β-carotene from hexane. The extraction capacity of different ionic liquids was screened, and the response surface method (RSM) based on central composite design (CCD) was used to optimize the experimental parameters. The results showed that the ionic liquid with a tetramethylammonium cation combined with an acetate anion extracted 63.09% of β-carotene.
In summary, although the organic solvent extraction method is simple to operate, the long extraction time is not conducive to β-carotene. Therefore, microwave and ultrasound should be introduced as auxiliary tools to shorten the extraction time.
2.2 Microwave-assisted extraction method
Traditional heating is based on heat transfer, which transfers the heat from the heat source to the sample. However, microwave heating does not require an intermediary medium, and the energy is introduced directly into the sample without a medium. Non-polar solvents do not absorb microwave energy, so in order to speed up the extraction, polar solvents are often added to non-polar solvents. Polar molecules receive microwave radiation energy and generate a thermal effect through molecular dipole rotation collisions at a frequency of 2.45 billion times per second [34]. In the microwave field, the difference in microwave absorption capacity causes some regions of the matrix material or some components of the extraction system to be selectively heated, thereby causing the extracted material to separate from the matrix or system and enter the extractant with a smaller dielectric constant and relatively poor microwave absorption capacity [35].
Hiranvarachat et al. [36] compared the extraction of β-carotene from carrots that had been pretreated with immersion in citric acid at pH 5, boiling water, and boiling citric acid solution at pH 5 for 1–1.5 min, with no pretreatment, and then extraction with microwave-assisted mixed solvents (50% hexane, 25% ethanol, with an azeotrope close to 58 °C) was used to extract β-carotene. The solid-liquid ratio was 2:75 (g/mL). The results showed that the amount of β-carotene in acid-pickled carrots was 23. 10mg/100 g, 29 . 74mg/100 g for the water-treated, 32 . 08mg/100 g for the boiling acid-treated, and 23 . 26mg/100 g for the untreated, indicating that the highest amount of β-carotene is found in the boiled citric acid-treated carrots. Because treating with low acid (pH 5) can destroy the polysaccharides in the plant cell walls, such as pectin and hemicellulose, without affecting the degradation of β-carotene, the β-carotene content can be increased [37].
However, microwave-assisted extraction can only be processed with lower power and shorter time. Excessive power or prolonged microwave processing time will cause the temperature of the extraction solution to rise, damaging the structure of β-carotene. Hiranvarachat et al. [38] used intermittent microwave extraction to extract β-carotene from carrot residue based on a previous experiment. The temperature of the condensed water was 4 °C, the intermittent rate was 1/4, microwave energy/solvent to sample ratio of 180 W/75 mL: 2 g, 300 W/150 mL: 2 g, the amount of β-carotene was 126 and 136 mg/100 g, respectively, proving that intermittent microwave extraction of β-carotene from carrots is better than continuous extraction.
2.3 Ultrasonic-assisted extraction method
Ultrasonic extraction technology is based on the cavitation effect of ultrasound to enhance the damage to the plant cell wall, thereby increasing the contact surface between the solvent and the analyte, so as to achieve the extraction effect. This technology can promote and accelerate the extraction process, avoid the damage of high temperature to the effective ingredients in the raw materials, make the effective ingredients relatively easy to separate and achieve more ideal extraction results than conventional extraction. Compared with traditional extraction methods, ultrasonic-assisted extraction technology can shorten the extraction cycle, improve product quality, and has the advantage of high extraction efficiency. Ultrasonic-assisted extraction technology can destroy the structure of cell walls and accelerate the dissolution of pigments[39].
Carail et al. [40] explored the effects of ultrasonic power, ultrasonic time, and ultrasonic temperature on the structure of β-carotene and found that as ultrasonic time increases, the energy obtained from the extraction solvent by ultrasonic waves is converted into heat due to the mechanical and thermal effects of ultrasonic waves, which causes the temperature of the extraction system to rise, the more serious the degradation of β-carotene. Purohit et al. [41] compared the extraction effects of an ultrasonic transducer and an ultrasonic bath on β-carotene from carrot residue. Under the ultrasonic transducer, the ultrasonic time was 50 min, 50 °C, 100 W, 60% duty cycle, and the solid-to-solvent ratio was 0. 3:20 (g/mL), the maximum extraction rate of β-carotene was 83.32%; in an ultrasonic bath, the maximum extraction rate of β-carotene was 64.66% with an ultrasonic time of 50 min, 50 °C, 180 W, 40 kHz.
Sun et al. [42] established a kinetic degradation equation for all-trans β-carotene and explored its stability during ultrasonic-assisted extraction with organic solvents. They found that β-carotene is very unstable in dichloromethane. The kinetic degradation equation shows that all-trans β-carotene undergoes a first-order degradation reaction at -5~15 ℃ in dichloromethane and a second-order degradation reaction at 25 ℃. The results indicate that dichloromethane is not suitable for the extraction of β-carotene under ultrasound. Li et al. [43] used sunflower oil as a solvent to extracted β-carotene from fresh carrots using ultrasound-assisted extraction. The ultrasound parameters were optimized using a response surface method and compared with the traditional hexane as the extraction solvent. The results showed that the oil-to-solid ratio was 2:10, the ultrasound intensity was 22. 5 W · cm-2, ultrasonic time 20 min, ultrasonic temperature 40 ℃, β-carotene amount 334.75 mg/L, which is greater than the amount extracted by hexane (321.35 mg/L), indicating that ultrasonic waves are effective and the process complies with the six principles of green extraction.
2.4 Microemulsion method
A microemulsion is a thermodynamically stable, isotropic, transparent or translucent dispersion system formed by two immiscible liquids. Microemulsions are composed of one or two liquid droplets stabilized microscopically by a surfactant interfacial film. Due to the thermodynamic stability of microemulsions, they can prevent the oxidation of carotenoids, thereby improving extraction efficiency. Microemulsions can be used to prepare functional β-carotene for food [44], but there are also reports of using the microemulsion method to extract β-carotene from carrots. For example, Roohinejad et al. [45] used an oil-in-water microemulsion as a medium for extracting β-carotene from carrots. plus pre-treatment with a pulsed electric field. The optimal extraction process parameters were determined using a microemulsion pseudo ternary phase diagram method. The results showed that the extraction time was 49.4 min, the temperature was 52.2 °C, the ratio of carrot to microemulsion was 1:70 (W/W), the β-carotene loading was 19.6 μg/g, the polydispersion index (PDI) was 0.27 and the particle size was 74 nm. The extraction efficiency was higher than 100% hexane or 100% glycerol monolaurin oil, which proves that the use of oil-in-water microemulsions can be used as a β-carotene extraction medium.
2.5 Accelerated solvent extraction
Accelerated solvent extraction is an automated pre-treatment technique developed in recent years. The extraction principle is as follows: by increasing the temperature (50–200 °C) and pressure (500–3000 psi), the van der Waals forces, hydrogen bonds and dipole moments between the solvent and the matrix are destroyed, the viscosity of the solvent is reduced, the surface tension of the solvent, solvent and substrate surface tension, increase the contact area between the analyte and the solvent, and enhance the solute diffusion efficiency, in order to improve the extraction efficiency. Compared with the commonly used Soxhlet extraction, ultrasonic extraction, microwave extraction and other methods, accelerated solvent extraction has the advantages of short extraction time (generally 15 min), low solvent consumption (only 1.5 mL solvent is required for 1 g sample), high extraction efficiency, high safety and high degree of automation [46]. 5 mL solvent), high extraction efficiency, high safety and high degree of automation, etc. [46]. However, the price of the accelerated solvent extractor is higher than that of the general microwave and ultrasound.
Saha et al. [47] used accelerated solvent extraction to extract carotenoids from carrots. The experimental Hildebrand solubility parameter was used to predict the selection of three different solvent combinations: acetonitrile and hexane in a ratio of 3:5, ethanol and hexane in a ratio of 4:3, and ethanol, hexane and acetonitrile in a volume ratio of 2:3:1 (all volume ratios) were selected for prediction. The extraction effects at different extraction temperatures of 40, 50, and 60 °C and times of 5, 10, 15min. After optimization, it was concluded that the extraction agent is a three-component mixture, the temperature is 60℃, the time is 15min, and diatomaceous earth is added as a desiccant during the extraction process (carrot: diatomaceous earth is 4:1).
2.6 Enzyme dissolution and extraction method
The combined treatment of cellulase and pectinase can effectively increase the extraction rate of β-carotene. This is because the plant cell wall is an extremely complex system, mainly composed of a cellulose skeleton, which is filled with a large amount of substances such as hemicellulose, pectin, and lignin. These substances are intermingled and mixed to form an extremely complex structural system. When improving the extraction rate of β-carotene in carrots, the double resistance of the cell wall and the intercellular substance must be overcome. Cellulase alone is difficult to completely lyse the cell wall. Combined treatment with pectinase can reduce the mass transfer resistance of the cell wall and intercellular substance, which is a mass transfer barrier to the diffusion of active ingredients from the inside of the cell to the extraction medium, and improve the extraction rate of active ingredients [48].
Ma et al. [49] studied the pretreatment of carrot juice with different enzymes to extract the carotenoids inside. After single factor experiments and orthogonal optimization, the experimental parameters were obtained: at a temperature of 45 °C, pH 5, reaction time 120 min, 1. 5% pectinase to extract β-carotene was 69.1 μg/mL, while at 50 °C, pH 5, reaction time 60min, 1. 5% cellulase to extract β-carotene was 68.7 μg/mL, and the addition of a single enzyme to the system in turn can also significantly increase the β-carotene content. However, when pectinase and cellulase are added simultaneously in equal proportions, the system shows antagonistic effects. According to analysis, because pectinase accounts for most of the complex enzymes used in industry, and cellulase only a small proportion, the treatment results with the two enzymes in the same proportion are not good. Enzymes are expensive, so there have been few reports of their application in this area in recent years.
2.7 Supercritical fluid extraction
The critical point of a substance is used, above which the interface between the gas and liquid phases disappears and the two phases become a homogeneous mixture. This fluid is called a supercritical fluid. In the supercritical state, supercritical fluid is brought into contact with the substance to be separated, so that the components with different solubility, boiling point and molecular weight can be selectively extracted and separated. The density and dielectric constant of supercritical fluid will increase with the increase of pressure in a closed system. By using programmed pressure increase, molecules with different polarities can be extracted step by step [50]. However, supercritical fluids are rare and demanding, which makes them not very widely used in various applications. Mustafa et al. [51] used supercritical CO2 to extract β-carotene from carrots, using vegetable mineral essence oil as a cosolvent to increase the extraction yield. The results showed that at 40min, 400bar, 60 ℃, the CO2 flow rate was 5mL/min, the mineral oil flow rate was 0.2mL/min, and the β-carotene extraction yield was about 270 μg/g dry weight and 35 μg/g wet weight.

3 Outlook
There are still some problems with the current extraction of β-carotene from carrots: (1) In terms of environmental safety and its application in food and cosmetics, the use of low-toxicity solvents to extract β-carotene from carrots has obvious advantages over conventional solvents. However, the generally longer extraction time (1–6 h) can cause degradation of β-carotene [52], and the source of low-toxicity solvents is more expensive than that of general solvents; (2) β-carotene is easily degraded and has a variety of isomers, and it is difficult to accurately quantify it due to the lack of standards; (3) ultrasonic-assisted extraction, accelerated liquid extraction, microwave-assisted extraction, enzyme-assisted extraction and supercritical fluid extraction are fast and effective, however, these techniques are still in the laboratory stage and industrialized large-scale applications are still not very common.
Reference:
[1]BBC Research. The g lobal makert for carotenoids[R/OL] . https://www. reportlinker. com/p096628/The-Global-Market- for-Carotenoids . html , 2017-08-22.
[2]Berman J , Zorrilla-López U , Farré G , et al. Nutritionally important carotenoids as consumer products[J] . Phytochemistry Reviews , 2015 , 14:727-743.
[3] Agricultural Marketing Resource Center. Carrots[R/OL] . http:// www. agmrc. org/,2017-08-22.
[4] Roszkowska B , Pilat B , Tańska M. Comparison of chemical composition of carrot roots of orange , purple and white colour[J] . Nauka Przyroda Technologie , 2015 , 9(4) :3-10.
[5] Attokaran M. Carrot[ M] . Natural Food Flavors and Colorants : John Wiley & Sons Ltd. , 2017:115-118.
[6]Datt S K , Karki S , Thakur N S , et al. Chemical composition , functional properties and p rocessing of carrota review[J] .Journal of Food Science and Technology , 2012 , 49 (1) : 22- 32.
[7] MendelováA , Mendel L , Fikselová M , et al. The dynamics of changes in nutritionally significant ing redients of carrot j uice after the p asteurization [J] . Acta Horticulturae et Regiotecturae , 2016 , 19 :8-12.
[8] Heinonen M I. Carotenoids and p rovitamin : a activity of carrot (Daucus carota L. ) cultivars [J] .Journal of Agricultural and Food Chemistry, 1990 , 38(3) :610-611.
[9] Nowacka M , Wedzik M. Effect of ultrasound treatment on microstructure , colour and carotenoid content in fresh and dried carrot tissue[J] . Applied Acoustics , 2016 , 103 : 163- 171.
[10] Gul K , Tak A , Singh A K , et al. Chemistry , encapsulation , and health benefits of β-carotene— a review [J] . Cogent Food & Agriculture , 2015(1) :1-12.
[11]Sanchez C , Baranda A B , Martinez I M. The effect of high pressure and high temperature p rocessing on carotenoids and chloro p hylls content in some vegetables [J] . Food Chemistry, 2014 , 163 :37-45.
[12] Wang Di, Liu Fuguo, Gao Yanxiang. Research progress of β-carotene emulsion [J]. China Food Additives, 2015(3): 170-177.
[13] Zhou Q X, Yang L. The effect of the combined effect of microemulsification and antioxidants on the stability of β-carotene [J]. Beverage Industry, 2017, 20(1): 3-8.
[14] Gurak P D , Mercadante A Z , Miret M G , et al. Changes in antioxidant capacity and colour associated with the formation of beta-carotene epoxides and oxidative cleavage derivatives[J] . Food Chemistry, 2014 , 147:160-169.
[15] Oxley A , Berry P , Taylor G , et al. An LC/MS/MS method for stable isotope dilution studies of beta-carotene bioavailability , bioconversion , and vitamin A status in humans[J] . Journal of Lipid Research , 2014 , 55 :319-323.
[16]Priyadarshani A M. A review on factors influencing bioaccessibility and bioefficacy of carotenoids[J] . Food Science and Nutrition , 2017 , 57(8) :1710-1717.
[17] Khoo H E , Prasad K N , Kong K W , et al. Carotenoids and their isomers : color p igments in fruits and vegetables[J] . Molecules , 2011 , 16(2) :1710-1738.
[18] Mueller L , Boehm V. Antioxidant activity of beta-carotene compounds in different in vitro assays[J] . Molecules , 2011 , 16(2) :1055-1069.
[19] Zhao Longgang, Zhang Qingli , Zheng Jiali , et al. Dietary , circulating beta-carotene and risk of all-cause mortality : a meta-analysis from prospective studies[J] . Scientific Reports , 2016(6) :1-8.
[20] Haskell M J . The challenge to reach nutritional adequacy for vitamin A : beta-carotene bioavailability and conversion- evidence in humans[J] . The American Journal of Clinical Nutrition , 2012 , 96(5) :193-203.
[21] Wang Zhixu , Yin Shian , Zhao Xianfeng , et al. β-Carotene- vitamin A equivalence in Chinese adults assessed by an isotope dilution technique [J] . British Journal of Nutrition , 2007 , 91(1) :121.
[22] Kim Y S , Lee H A , Lim J Y , et al. beta-carotene inhibits neuroblastoma cell invasion and metastasis in vitro and in vivo by decreasing level of hypoxia-inducible factor-1alpha[J] . Journal of Nutritional Biochemistry, 2014 , 25(6) :655-664. [23] Relevy N Z , Bechor S , Hararin A , et al. The inhibition of macrophage foam cell formation by 9-cis beta-carotene is driven b y BCMO1activity[J] . PloS one , 2015 , 10(1) :1-15.
[24] Wachea Y , Deratuld A B , Lhuguenot J C , et al. Effect of cis/trans isomerism of β-carotene on the ratios of volatile compounds p roduced during oxidative deg radation[J] . J . Agric. Food Chem. , 1984 , 51(7) :1984-1987.
[25] Tanaka T , Shnimizu M , Moriwaki H. Cancer chemoprevention by carotenoids[J] . Molecules , 2012 , 17(3) :3202-3242.
[26] Xu Congcong , Li Yunfei , Wang Liping , et al. Evaluating and correlating the mechanical , nutritional , and structural properties of carrots after multip le freezing/thawing p rocessing[J] . Journal of Food Science and Technology , 2017 , 54(8) :2251-2259.
[27]Fikselova M , ilhar S , Marecek J . Extraction of carrot (Daucus carota L. ) carotenes under different conditions[J] . Czech J . Food Sci. , 2008 , 26(4) :268-274.
[28] Fan Hongbo. Research on the application of O/W microemulsion in the extraction of pueraria root [D]. Jilin: Jilin University, 2015.
[29] Corteas C , Esteve M , Friagola A , et al. Identification and quantification of carotenoids including g eometrical isomers in fruit and vegetable juices b y liquid chromato graphy with ultraviolet-diode array detection[J] .JournalofAgricultrual and Food Chemistry, 2004 , 52(8) :2205.
[30] Nowak A M , Swiderski A , Kruczek M , et al. Content of carotenoids in roots of seventeen cultivars of Daucus carota L. [J] . Communication , 2012 , 59(1) :139-141.
[31]Carrilho K A , Cepeda A , Fente C , et al. Review of methods for analysis ofcarotenoids[J] . Trends inAnalytical Chemistry , 2014 , 56 :49-73.
[32] Varón Y E , Tixier F A , Balcells M , et al. Is it possible to substitute hexane with green solvents for extraction of carotenoids A theoretical versus experimental solubility study[J] . The Royal Society of Chemistry , 2016 , 33 (6) : 27750-27759.
[33] Rajabi M S , Moniruzzaman M , Mahmood H , et al. Extraction of β-carotene from organic p hase using ammonium based ionic liquids aqueous solution [J] . Journal of Molecular Liquids , 2017 , 227:15-20.
[34] Jin Qinhan, Dai Shushan, Huang Kama. Microwave Chemistry [M]. Beijing: Science Press, 1999: 166.
[35] Chen Meng, Yuan Dongxing, Xu Pengxiang. Research progress of microwave extraction [J]. Journal of Analytical Testing, 1999(2): 83-87.
[36] Hiranvarachat B , Devahastin S , Chiewchan N , et al. Structural modification b y different pretreatment methods to enhance microwave-assisted extraction of β-carotene from carrots [J] . Journal of Food Engineering, 2013 , 115(2) :190-197.
[37] Rich G T , Travis F A , Parker M L. Low pH enhances the transfer of carotene from carrot juice to olive oil[J] . Lipids , 1998 , 33(10) :985-998.
[38] Hiranvarachat B , Devahastin S. Enhancement of microwave- assisted extraction via intermittent radiation : extraction of carotenoids from carrot peels[J] .Journal of Food Engineering , 2014 , 126 :17-26.
[39] Jin Si, Ma Kongjun. Research progress on ultrasonic-assisted extraction of carotenoids [J]. Food Research and Development, 2017(9): 192-197.
[40] Carail M , Tixier A F , Meullemiestre A , et al. Effects of high power ultrasound on all-E-beta-carotene, newly formed compounds analysis b y ultra-high-p erformance liquid chromato graphy- tandem mass spectrometry[J] . Ultrasonics Sonochemistry , 2015 , 26 :200-209.
[41]Purohit A J , Gogate P R. Ultrasound-assisted extraction of β-carotene from waste carrot residue : effect of operating parameters and type of ultrasonic irradiation[J] . Separation Science and Technology, 2015 , 50(10) :1507-1517.
[42]Sun Yujing, Ma Guangpeng, Ye Xingqian , et al. Stability of all-trans-β-carotene under ultrasound treatment in a model system : effects of different factors , kinetics and newl y formed compounds[J] . Ultrasonics Sonochemistry , 2010 , 17(4) :654-661.
[43] Li Yong , Tixier A F , Tomao V , et al. Green ultrasound- assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent[J] . Ultrasonics Sonochemistry, 2013 , 20:12-18.
[44] Cao Yanping, Xu Duo Xia, Yuan Fang, et al. Research progress on functional food pigments emulsions [J]. China Food Additives, 2014(3): 183-187.
[45] Roohinejad S , Oey I , Everett D W , et al. Evaluating the effectiveness of β-carotene extraction from p ulsed electric field-treated carrotpomace using oil-in-water microemulsion[J] . Food Bioprocess Technol , 2014(7) :3336-3348.
[46] Niu Gai, Deng Jianchao, Li Haolai, et al. Accelerated solvent extraction and its application in food analysis [J]. Food Industry Science and Technology, 2014, 35(1): 375-380.
[47] Saha S , Walia S , Kundu A , et al. Optimal extraction and fingerprinting of carotenoids by accelerated solvent extraction and liquid chromatography with tandem mass spectrometry[J] . Food Chemistry, 2015 , 177 :369-375.
[48] Zhang Bo, Liu Wu, Guo Qiang, et al. Research progress on the extraction process of β-carotene [J]. Chemical Technology Market, 2009, 32(4): 34-37.
[49] Ma Tingting, Luo Jiyang, Tian Chengrui , et al. Influence of enzyme liquefaction treatment on maj or carotenoids of carrot (Daucus carrot L. ) juice[J] . Journal of Food Processing and Preservation , 2016 , 40(6) :1370-1382.
[50] Zhang Hongying, Yao Yuanhu, Yan Xueming. Supercritical fluid extraction and separation technology and its application [J]. Journal of Capital Normal University (Natural Science Edition), 2016, 37(6): 50-53.
[51] Mustafa A , Verendel J , Turner C , et al. Evaluation of oxidation stability of refined mineral oil enriched with carotenoids from carrot using supercritical carbon dioxide extraction[J] . Industrial & Engineering Chemistry Research , 2014 , 53(49): 19028-19033.
[52]Sharmin T , Ahmed N , Hossain A , et al. Extraction of bioactive compound from some fruits and vegetables (pomegranate peel , carrot and tomato ) [J] . American Journal of Food and Nutrition , 2016 , 4(1) :8-19.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese