Study on the Benefits of Artichoke Leaf Extract
Artichoke (Cynara scolymus L.) is a perennial herb in the Asteraceae family. Also known as the vegetable thistle, European thistle, thistle, lotus lily, and French lily, it is known as the “king of vegetables” and is native to the Mediterranean coast. In the 4th century BC, the artichoke was already appreciated by the ancient Egyptians, Greeks and Romans as a medicinal and edible Asteraceae plant, and made a significant contribution to the agricultural economy of the Mediterranean. It was introduced to China from France in the 19th century and has been cultivated for more than 100 years. Artichoke cultivation in China is increasing year by year[1] and is mainly distributed in Shanghai, Zhejiang, Yunnan, Shandong, Hunan and Beijing. Artichoke buds are edible and their extracts have long been used in folk medicine[1] .
Artichoke extract is rich in polyphenols[2-4] . In vitro, it has antioxidant[5] , antibacterial[6] , anticancer and antitumor[7] , hepatoprotective[8] and hypocholesterolemic[9] effects. Experiments have shown that standardized artichoke extract capsules can clinically lower plasma cholesterol[10] in patients with hypercholesterolemia, reduce irritable bowel syndrome [11], lose weight [12], lower the glucose metabolism parameters of diabetic patients [13], and improve digestion [14-15]. In order to promote the comprehensive development and utilization of this plant in China, this paper intends to summarize the research results on its polyphenolic compounds, extraction methods, analytical determination methods, and biological activities, to provide a reference for its development and utilization.

1 Polyphenolic substances in artichoke extract
The phenolic compounds in artichoke extract mainly include flavonoids (including anthocyanins), phenolic acids (ferulic acid derivatives, quinic acid derivatives, and caffeic acid quinic acid derivatives), and coumarins.
1.1 Flavonoids
The flavonoids in artichoke extract are mainly luteolin glycosides, apigenin glycosides and anthocyanins.
Romani et al. [16] analyzed the phenolic compounds of different parts (leaves, outer bracts, flower buds and stems) of the cultivars Violetto di Toscana and Terom, which are widely cultivated in Italy, by HPLC-DAD-MS. The results showed that the leaves had the highest flavonoid content compared to other parts. Dranik et al. [17] were the first to extract luteolin 7-O-glucoside, apigenin 7-O-glucoside and luteolin 3-O-glucoside from artichoke leaves. Luteolin compounds are the main components of artichoke leaves, while the main components of other parts are not luteolin compounds. Mulinacci et al. [18] found that luteolin 7-O-glucoside and luteolin 7-O-rutinoside are the two flavonoids with the highest content in artichoke leaves. Orlovskaya et al. [19] detected that luteolin 7-O-glucoside accounted for 35.19% of the phenolic compounds in artichoke leaves.
Sarawek et al. [20] also confirmed that the main flavonoid in artichoke leaf extract is luteolin-7-O-glucoside, followed by apigenin-7-O-glucuronide. Gebhardt et al. [21] detected apigenin-7-O-glucuronide in the leaves. Schütz et al. [22] studied the flavonoids in artichoke heads, juice and pomace and found that apigenin-7-O-glucuronide was the main flavonoid in all the samples studied, 1,002 mg/kg · DM (dry matter) in artichoke heads and 1,318 mg/kg · DM in artichoke pomace. Wang et al. [5] also detected apigenin-7-O-glucuronide in the outer bracts and heads; Lombardo et al. [4] also demonstrated that apigenin-7-O-glucuronide is the main flavonoid compound, with a content of 6298 mg/kg · dry matter of the flower receptacle of Romanesco clone C3 (variety).
Wang et al. [5] analyzed the polyphenolic compounds in artichoke leaves, mature bracts and immature bracts using HPLC and detected, in addition to luteolin, the two most common flavonoids in artichoke, luteolin-7-O-glucoside and luteolin-7-O-rutinoside, as well as apigenin-7-O-glucuronide in the outer bracts and heads, apigenin-7-O-glucuronide in the outer bracts in small amounts, and apigenin-7-O-malonyl-β-D-glucoside in the young leaves.

Lombardo et al. [4] detected naringenin-7-O-glucoside and naringenin-7-O-rutinoside as minor phenolic compounds in artichoke. El-Negoumy et al. [23] also detected naringenin, naringenin-7-O-glucoside, naringenin-7-O-rutinoside and apigenin-7-O-glucoside as well as apigenin-7-O-rutinoside, apigenin-7, 4'-O-diglucoside and luteolin-3', Hinou et al. [24] reported that there are compounds such as apigenin, apigenin-7-rutinoside and rutin in artichoke leaves. Orlovskaya et al. [19] reported that fresh artichoke leaves contain small amounts of the phenolic compounds dihydroquercetin, myricetin, isoquercitrin, hyperoside, and hesperidin, and that the water extract of dried artichoke leaves contains 1.27% of the phenolic compound robinetin.
Phenolic compounds are generally found in bound form. Lattanzio et al. [25] investigated the changes in phenolic compounds in artichoke heads during growth and refrigeration. The results showed that phenolic substances are all bound in fresh, healthy heads. Only trace amounts of free apigenin and luteolin were detected in fresh heads, while measurable amounts of apigenin and luteolin were found.
Compared to studies on phenolic acids and flavonoids, there are very few data on anthocyanins. Aubert et al. [26] identified the following anthocyanins in artichoke flowers, bracts and leaves: caryophyllene 3-caffeoyl locustoside-5-glucoside, caryophyllene 3-locustoside, caryophyllene 3-glucoside, caryophyllene 3-caffeoyl locustoside and caryophyllene 3-caffeoyl glucoside. Schütz et al. [27] identified 13 anthocyanins. These included 10 delphinidin derivatives, 2 peonidin derivatives and 1 cyanidin derivative. Of the 13 anthocyanins, 6 had a glucopyranosyl-propionate substituent and 1 was a glucoside.
1.2 Caffeoylquinic acid compounds
Caffeoylquinic acids are a group of phenolic acids that are composed of quinic acid and varying numbers of caffeic acid linked by ester bonds [28]. Artichoke is a rich source of caffeoylquinic acids, which accumulate caffeic acid (3,4-dihydroxycinnamic acid) residues, with mono- and di-caffeoylquinic acids as the main chemical components. The main mono-caffeoyl compounds in artichoke are 1-O-caffeoylquinic acid, 3-O-caffeoylquinic acid (neochlorogenic acid), 4-O-caffeoylquinic acid (cryptochlorogenic acid) and 5-O-caffeoylquinic acid (chlorogenic acid), chlorogenic acid is the most abundant [3]; the main components of di-caffeoylquinic acid are 1,3-dicaffeoylquinic acid, 1,4-dicaffeoylquinic acid, 1,5-dicaffeoylquinic acid, 3,4-O-dicaffeoylquinic acid and 3, 5-O-dicaffeoylquinic acid, of which 1,5-dicaffeoylquinic acid is the most abundant, followed by 3,4-O-dicaffeoylquinic acid [1].
Cynarin is the most commonly cited caffeoylquinic acid derivative in artichoke extract (heads and leaves) and has liver-protecting, choleretic and cholesterol-lowering effects. Panizzi et al. [29] first isolated the cynarin monomer from artichoke leaves in 1954 and resolved it as 1,4-O-dicaffeoylquinic acid. In 1965, Panizzi re-analyzed its structure as 1,5-O-dicaffeoylquinic acid, and according to the current nomenclature for organic compounds, its name is 1,3-O-dicaffeoylquinic acid [1]. Zhu et al. [30] also obtained artichoke extract from the extract of artichoke leaves. Cynarin is very active but not abundant [1] and is therefore sometimes not detected. Lattanzio et al. [31] showed that the content of cynarin in artichoke heads was 61.2 mg/100 g · DW, 0.07 to 0.42 times that of several other di-caffeoylquinic acids. Azzini et al. [32] studied the phenolic composition of artichoke heads and detected artichokein only after cooking.
Chlorogenic acid is the main monocaffeoylquinic acid compound in artichokes. Lombardo et al. [4] studied the polyphenol composition of artichokes and found that each kilogram of 'Violetto di Sicilia' contains 14,841 mg of dry matter in the bracts. Schütz et al. [22] studied the caffeic acid compounds in artichoke heads, juice and pomace and showed that chlorogenic acid is abundant, second only to 1,5-O-dicaffeoylquinic acid, 3143 mg/kg · DM in artichoke heads and 2033 mg/kg · DM in pomace.
Schütz et al. [22] found that 1,5-O-dicaffeoylquinic acid is the main hydroxycinnamic acid in their study of caffeoylquinic acids in artichoke heads, juice and pomace. in artichoke heads, it was found to contain 3 890 mg/kg · DM, and in pomace, the content was 3 269 mg/kg · DM. In the juice, 1, 3-O -dicaffeoylquinic acid (artichoke) was dominant, which may be caused by isomerization during processing.
The content of caffeoylquinic acid derivatives in artichoke tissue depends on the physiological stage of the tissue. Wang et al. [5] studied the antioxidant phenolic compounds in the leaves and heads of three varieties of artichoke (Globe, Imperial Star and Violet), and the results showed that the leaves contained the highest concentration of total phenols, and the immature head bracts had a higher ratio than the mature head bracts. Among the three artichoke varieties, the highest concentration of phenols, while the violet variety had the lowest.
The main phenolic compounds in artichoke undergo transformation during storage and processing. Lattanzio et al. [25] showed that the healthy heads of artichoke stored at 20 °C for 2 weeks or at 4 °C for 1 month had a significant increase in caffeic acid, with the most significant increase at 20 °C. A damaged head (internal blackening) stored at 20 °C for 2 weeks showed a decrease in caffeic acid and most other phenolic substances. In severely damaged heads, caffeic acid was found to be less than half of the total amount present in fresh marketable heads during the same period of storage. However, the decrease in caffeic acid in damaged heads stored at 4 °C for 1 month was slower.
Gil-Izquierdo et al. [33] showed that the content of total phenol, chlorogenic acid and 1,4-di-caffeoylquinic acid + 4,5-dicaffeoylquinic acid in the bracts of artichokes increased after storage, especially at 2, 5 and 7 °C, while 1, 5-dicaffeoylquinic acid + 3,5-dicaffeoylquinic acid decreased from 260 mg/kg to 150 mg/kg, indicating that phenolic compounds have undergone transformation. Azzini et al. [32] found that after artichoke was cooked, a slight increase in chlorogenic acid content, and an increase in monocaffeoylquinic acid and dicaffeoylquinic acid.
1.3 Coumarin compounds
Hinou et al. [24] reported that 7-hydroxy-6-methoxycoumarin and 7-hydroxy-6-O-β-glucopyranosyl-coumarin are present in artichoke leaves. Orlovskaya et al. [19] reported 4-hydroxycoumarin. Vigh et al. [34] also reported that dried artichoke leaves contain 7-hydroxy-6-methoxycoumarin.
1.4 Other phenolic acids
Orlovskaya et al. [19] showed that among the phenolic compounds in fresh artichoke leaves, caffeic acid accounted for 38.55% and arbutin for 9.31%. The water extract of dried artichoke leaves contained gallic acid (23.48%), chicoric acid (5.86%) and ferulic acid (5.54%). Azzini et al. [32] studied the absorption and metabolism of bioactive molecules in artichoke and found that 8 h after oral administration of artichoke, the blood levels of dihydrocaffeic acid and dihydroferulic acid increased significantly, confirming the absorption and utilization of hydroxycinnamic acid metabolites after ingestion of artichoke.
2 Extraction of polyphenolic substances from artichoke
Common extraction methods for polyphenolic compounds include solvent extraction, ultrasound-assisted extraction, microwave-assisted extraction, enzyme-assisted extraction, and supercritical fluid extraction. Its main compound, chlorogenic acid, is easily soluble in polar solvents such as ethanol, acetone and methanol, slightly soluble in ethyl acetate, and hardly soluble in lipophilic organic solvents such as chloroform, ether and benzene. Due to its own instability, it cannot be extracted at high temperatures, under strong light or for long periods of time.
During the extraction of polyphenolic compounds, solvent extraction methods can be divided into hot water extraction and alcohol (methanol or ethanol) extraction, depending on the solvent used. Hot water extraction is not commonly used because it causes the extract to contain more impurities. At present, for the extraction of polyphenolic compounds from artichoke, an alcohol solvent of an appropriate concentration can be selected according to the composition of the polyphenolic compounds.
Song Shuhui et al. [35] used methanol, ethanol and other solvents to ultrasonically extract the polyphenols from artichoke. The results showed that the optimal conditions were an extraction power of 650 W, an extraction time of 20 min, an extraction temperature of 30 °C, liquid-to-solid ratio of 1:20 (g/mL), 70% ethanol as the solvent, and the polyphenol extraction rate under these conditions was 4.06%.
Due to the complex polyphenol compounds in artichokes, there is not just one method for extracting them. Usually, two or more methods are combined to improve the extraction rate. Zhang Jun et al. [36] used a method combining microwave-assisted and solvent extraction, and determined the optimal conditions through single-factor experiments and orthogonal experiments: a material-to-liquid ratio of 1:8 (g/mL), an extraction liquid pH of 7, a microwave power of 700 W and a processing time of 90 s. Under these conditions, the polyphenol extraction rate of artichoke leaves can reach 2.27%. Zhao Youyi et al. [37] used a solvent reflux extraction method to investigate the factors affecting the extraction rate of flavonoids and phenolic acids (indicated by the caffeic acid content) in artichoke leaves. The optimal extraction process was 60% ethanol, liquid-to-material ratio 10:1 (mL/g), extraction for 2 hours three times. Under the optimal conditions, the average content of total phenolic acids was 5.01%, and the average extraction rate of total flavonoids and phenolic acids was 95.74%.
3 Qualitative and quantitative analysis of polyphenols in artichoke
The methods commonly used for the analysis and determination of polyphenolic compounds include spectrophotometry, high-performance liquid chromatography, and liquid chromatography-mass spectrometry. Spectrophotometry can be used to qualitatively and quantitatively analyze total phenols, while high-performance liquid chromatography and liquid chromatography-mass spectrometry can be used to qualitatively and quantitatively analyze the individual compounds after column separation.
3.1 Spectrophotometry
Spectrophotometry includes ultraviolet-visible spectrophotometry, the Folin-Ciocaileu colorimetric method, atomic absorption spectrophotometry, and the ferric tartrate spectrophotometry method [38]. Spectrophotometry can be used to determine the total phenol content in natural products [39]. Feng Li et al. [40] studied the total phenol content of three varieties of artichoke (Emperor, Green Gem, and German) to study the total phenol content. Fratianni et al. [41] used the Folin-Ciocaileu method to study the total phenol content of different parts of artichoke, and Rezazadeh et al. [42] used the method to study the effect of salinity on the phenolic composition of artichoke leaves.
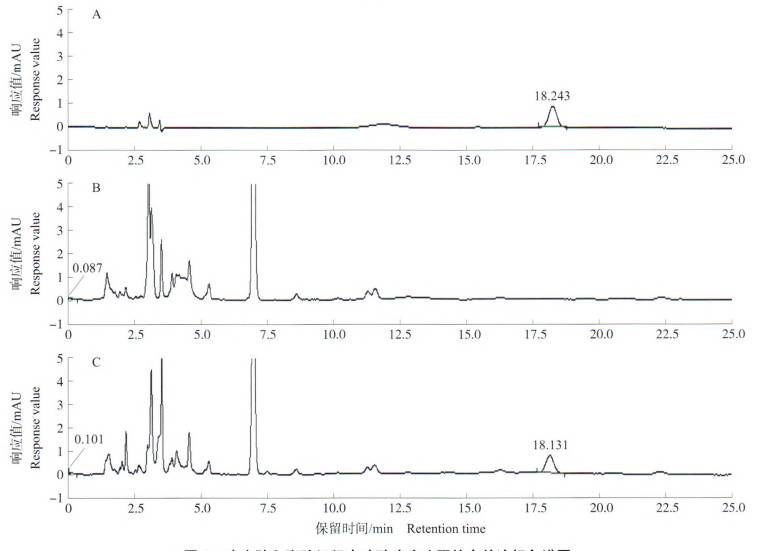
3.2 High-performance liquid chromatography
High-performance liquid chromatography is commonly used to separate polyphenolic compounds. Combined with the analysis of the content of individual compounds using standard samples, this method has the advantages of high sensitivity, a wide linear range, and rapid analysis [43]. Cao Peiqin et al. [44] established a high-performance liquid chromatography method for the simultaneous determination of chlorogenic acid (3-caffeoylquinic acid) and artichoke (1, 5-dicaffeoylquinic acid) in artichoke leaves by high performance liquid chromatography (HPLC), and the contents of chlorogenic acid and artichoside were determined to be 1.01% and 0.026%, respectively. Gil-Izquierdo et al. [33] used HPLC to determine that the total phenol content of the inner bracts was much higher than that of the outer ones (618, 74 mg/kg in fresh leaves, respectively).
Azzini et al. [32] used HPLC-DAD to determine the changes in polyphenol content in raw and cooked fresh artichoke heads. Zhang et al. [45] used UPLC method to determine the content of chlorogenic acid in artichoke leaves (mass fraction) to be 1.52%. Orlovskaya et al. [19] used liquid chromatography to determine the content of phenolic compounds in fresh artichoke leaves. The results showed that luteolin-7-glucoside 35.19%, rutin 0.08%, dihydroquercetin 0.91%, apigenin 8-C-β-D-glucoside 5.31%, 0.46% apigenin-8-C-glucoside, 0.01% hyperoside (quercetin 3-β-D-galactoside), 2.33% hesperidin, 0.88% 4-hydroxycoumarin, 0.10% chlorogenic acid, 6.88% neochlorogenic acid, 38.55% caffeic acid and 9.31% arbutin. In the water extract of dried artichoke leaves, no phenolic compounds such as dihydroquercetin, hyperoside, isoquercitrin, hesperidin, 4-hydroxycoumarin and arbutin were detected compared to fresh artichoke leaves. However, the newly detected phenolic substances were apigenin (0.89%), robinin (1.27%), gallic acid (23.48%), chicoric acid (5.86%) and ferulic acid (5.54%). The results confirmed that luteolin-7-glucoside is the main flavonoid compound in artichoke leaves.
3.3 Liquid chromatography-mass spectrometry
High performance liquid chromatography-mass spectrometry can be used to rapidly separate crude extracts of natural products, identify components based on fragment ion information, and perform quantitative analysis. It has the characteristics of high sensitivity, low sample consumption, and fast analysis. Lombardo et al. [4] used HPLC-DAD-ESI/MSn to identify 19 phenolic compounds, and Mulinacci et al. [18] used HPLC/MS to identify 4 mono-caffeoyl quinic acids, 5 di-caffeoyl quinic acids and 5 luteolin derivatives.
Luo et al. [46] used liquid chromatography-electrospray tandem quadrupole mass spectrometry (LC-MS/MS) to study the content of chlorogenic acid in canned artichoke foods. The highest content was found in canned artichoke hearts, with an average value of 625.701 μg/g, and the average value of artichoke inulin was 315.823 μg/g. Pandino et al. [47] analyzed the polyphenol distribution in different parts of different varieties of artichoke (Blanc Hyerois, Nobre, Tema 2000, Tempo F1, Tondo di Paestum, Violetto di Sicilia) using HPLC-DAD-MS/MS. The results show that the phenolic content varies significantly among varieties and parts. Among them, the chlorogenic acid content ranged from 2,278 mg/kg·DM (Tondo di Paestum) to trace amounts (No-bre); and the 1,5-O-dicaffeoylquinic acid content ranged from 83 mg/kg·DM (Blanc Hyerois) to 1,986 mg/kg·DM (Tondo di Paestum). Schütz et al. [27] performed mass spectrometry analysis on the extracts of the heads of four cultivars, and the results showed that the anthocyanin content varied greatly among different varieties, with a total content ranging from 8 to 1705 mg/kg·dry basis. Cyanidin 3-(6"-malonyl) glucoside was the main anthocyanin in all the samples analysed.
4 Bioactivity of the phenolic compounds in artichoke
4.1 Antioxidant activity
Oxygen free radicals are involved in the pathophysiology of many diseases, such as inflammation, ischemic heart disease, and cancer [48]. Artichoke may have good free radical scavenging ability due to the presence of polyphenolic compounds, which can prevent lipid peroxidation, inhibit hemolysis caused by hydrogen peroxide, and oxidation of cells by peroxides.
Song Shuhui et al. [49] used the DPPH method and the ABTS method to conduct in vitro antioxidant tests on artichoke leaf extracts. The results showed that the IC 50 value for the removal of DPPH · by artichoke leaf extract was (283.5±0.69) μg/mL, and the IC 75 value was (443.6±0.74) μg/mL. When the concentration of the extract was increased, the inhibition rate of the ABTS+ free radical gradually increased. Yang Haiying et al. [50] studied the antioxidant activity of artichoke leaf extract using the superoxide anion method. When the concentration of artichoke leaf extract was 0.8 mg/mL, the scavenging rate of superoxide anions by artichoke leaf extract reached 92.92%.
Studies on the substance basis of the activity of artichoke extract have shown that the antioxidant activity is related to the number of phenolic hydroxyl groups, and the number of polyhydroxyl groups indicates high antioxidant activity. The addition of a second hydroxyl group in the ortho or para position can also increase antioxidant activity. Wang et al. [5] used the DPPH method to test the activity of isolated compounds, and the activity was as follows: artichoke (2 adjacent hydroxyl groups per phenolic ring) > luteolin, luteolin-7-rutinoside (2 adjacent hydroxyl groups on one ring, only 1 hydroxyl group on the second ring) > chlorogenic acid, 1-caffeoylquinic acid (2 adjacent hydroxyl groups on the same phenolic ring) > apigenin-7-rutinoside, naringenin (2 hydroxyl groups on separate phenolic rings). Evcíková et al. [51] measured the activity of polyphenols using the DPPH method: di-caffeoylquinic acid > apigenin-7-glucoside > chlorogenic acid.
Jun et al. [52] identified the substance in artichoke that showed antioxidant activity as artichin by HPLC-MS and NMR (1 H and 13 C), and determined its antioxidant activity using the DPPH method and the ABTS method. EC 50 were 5. 56 and 15.83 μg/mL, respectively. Gil-Izquierdo et al. [33] showed that artichoke is an important source of natural antioxidants, with phenolic substances in the inner bracts of artichoke 10 times higher than those in the outer bracts. After harvest and storage, the total phenolics in the inner bracts were 618 mg/kg, chlorogenic acid was 143 mg/kg, 1,4-di-caffeoylquinic acid + 4,5-dicaffeoylquinic acid was 207 mg/kg, and 1,5-dicaffeoylquinic acid + 3,5-dicaffeoylquinic acid was 260 mg/kg.
Pérez-García et al. [53] found that artichoke leaf extract and the pure components artichoke, caffeic acid, chlorogenic acid and luteolin all reduced H 2 O 2 -induced oxidation of human leukocytes in a concentration-dependent manner, with an inhibition rate of 50% at a concentration of 10-100 μg/mL for artichoke leaf extract. The pure components tested showed the same inhibition levels at lower concentrations of 3.5–9.0 μg/mL: chlorogenic acid at 3.5 μg/mL (inhibition rate 66.1%), artichoke at 5.2 μg/mL (55.2%), 5.7 μg/mL for caffeic acid (55.9%) and 9.0 μg/mL for luteolin (51.6%).
4.2 Bacteriostatic activity
Vamanu et al. [54] optimized the extraction conditions using the polyphenol content as the evaluation index, and obtained a 75% ethanol extract of artichoke leaves. Bacteriostatic tests showed that The extract showed significant inhibitory activity against the test strains of harmless Listeria CMGB 218 and Bacillus cereus CMGB 215, with MICs of 5 mg/mL, but the MICs against other strains were 15 mg/mL. Yang Kesha [55] conducted a bacteriostatic test on crude polyphenols extracted from artichoke leaves by ethanol and purified polyphenols. The results showed that the MIC values of crude polyphenols against the test bacteria were 25.0, 22.5, 20.0, 32.5, 27.5, and 30.0 mg/mL, The purified polyphenols had stronger antibacterial activity against Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa, Lactobacillus and Candida albicans than the crude polyphenols. The MIC values of the purified polyphenols against the test bacteria were 10, 8, 12, 13, 12 and 13 mg/mL, respectively.
Zhu et al. [30] studied the antibacterial activity of extracts from three extraction solvents (chloroform, ethyl acetate and n-butanol) and the isolated monomeric compounds. Among them, the n-butanol extract showed the most significant antimicrobial activity against the three types of microorganisms (7 bacteria, 4 yeasts and 4 molds); the n-butanol extract was purified to obtain caffeoylquinic acid derivatives and four flavonoids, among which chlorogenic acid, artichoke, luteolin-7-rutinoside and luteolin showed relatively higher activity than other compounds; they were more effective against fungi than bacteria, with minimum inhibitory concentrations of 50–200 μg/mL.
4.3 Hepatoprotective effect
Artichoke is a member of the Asteraceae family that is used in Western medicine and as food. Its leaf extract has long been used in folk medicine, especially for liver disease[1] . Currently, the commercialized drug is mainly used to treat liver disease.
Using rat CCU poisoning as a test model, Speroni et al. [8] evaluated the hepatoprotective effect of artichoke extract by measuring lipid peroxidation, aspartate aminotransferase and alanine aminotransferase activity, and the results showed that the extract had a protective effect on bile flow and the liver. Gebhardt et al. [56] showed that when primary rat hepatocytes were exposed to tert-butyl hydroperoxide (t-BHP), the artichoke water extract reduced lipid peroxidation (malondialdehyde production) and cytotoxicity in the culture, indicating its antioxidant and hepatoprotective potential. Regular consumption of artichokes has therapeutic effects on chronic hepatitis, and most of these effects are attributed to artichoke extracts. Research on the liver-protecting properties of individual artichoke substances is limited to studies on artichinine. Adzet et al. [2] established an in vitro rat liver cell CCl4 poisoning model and demonstrated that artichinine has a significant protective effect on liver cells, as measured by GOT and GPT enzyme leakage.
4.4 Cholesterol-lowering and hyperlipidemia
Hyperlipidemia often leads to cardiovascular diseases such as atherosclerosis and coronary heart disease, which seriously threaten people's health. Indicators such as TG, TC, LDL-C and HDL-C can be used to monitor the condition. In recent years, research on artichoke at home and abroad has shown that its extract can lower cholesterol and reduce the risk of hyperlipidemia. Bundy et al. [10] conducted a randomized double-blind placebo-controlled trial, which showed that the artichoke leaf extract reduced total plasma cholesterol by an average of 4.2%, while the control group increased by an average of 1.9%. Yao Min et al. [57] further studied the therapeutic effect of artichoke extract on hyperlipidemia, as well as its main active substance luteolin, and confirmed that artichoke leaf extract can enhance the excretion of cholesterol and reduce cholesterol synthesis in the liver. Among them, luteolin inhibits cholesterol biosynthesis by 60%. Song Shuhui et al. [58] investigated the content of TG, TC, HDL, LDL, and MDA in the blood and liver, as well as the activities of lipoprotein lipase (LPL) and hepatic lipase (HL) in the liver, through animal experiments, and also found that artichoke leaf extract has a certain function of lowering blood lipids.
5 Prospects
At present, most health foods have added chemical additives, and the potential safety risks and harm to consumer health are self-evident. Artichoke has great potential for development in the field of health foods. On the one hand, if the existing results of research on artichoke polyphenols at home and abroad can be utilized, digested and put into production, it will generate huge economic benefits and promote the vigorous development of China's plant-derived natural antioxidant industry. On the other hand, with the increasing development of the medicinal value of artichokes by modern medicine and people's increasing awareness of the health benefits of artichokes, the demand for artichoke tea bags, artichoke health foods, artichoke dried powder and artichoke extracts will gradually increase.

Reference
[1] LATTANZIO V , KROON P A , LINSALATA V , et al. Globe artichoke : a functional food and source of nutraceutical ingredi- ents [J] . Journal of Functional Foods , 2009 , 1(2) : 131-144.
[2] ADZET T , CAMARASA J , LAGUNA J C. Hepatoprotective activity of polyphenolic compounds from Cynara scolymus against CCl4toxicity in isolated rat hepatocytes [J] . Journal of Natural Products , 1987 , 50(4) : 612-617.
[3] ADZET T , PUIGMACIA M. High-p erformance liquid chroma- tography of caffeoylquinic acid derivatives of Cynara scolymus L. leaves[J] . Journal of Chromatography A , 1985 , 348 : 447-453.
[4] LOMBARDO S , PANDINO G , MAUROMICALE G , et al. In- fluence of genotype , harvest time and plant part on polyphenolic composition of globe artichoke [Cynara cardunculus L. var. sco- lymus (L. ) Fiori][J] . Food Chemistry , 2010 , 119(3) : 1 175- 1 181.
[5] WANG Ming-fu , SIMON J E , AVILES I F , et al. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L. )[J] . Journal of agricultural and Food Chemistry , 2003 , 51(3) : 601-608.
[6] ZHU Xian-feng , ZHANG Hong-xun , LO R. Antifungal activity of Cynara scolymus L. extracts [J] . Fitoterapia , 2005 , 76 (1) : 108-111.
[7] MILEO A M , Di VENERE D , LINSALATA V , et al. Artichoke polyphenols induce apoptosis and decrease the invasive potential of the human breast cancer cell line MDA-MB231[J] . Journal of Cellular Physiology , 2012 , 227(9) : 3 301-3 309.
[8] SPERONI E , CERVELLATI R , GOVONI P , et al. Efficacy of different Cynara scolymus p reparations on liver complaints[J] . Journal of Ethnopharmacology , 2003 , 86(2) : 203-211.
[9] SHIMODA H , NINOMIYA K , NISHIDA N , et al. Anti-hyp er- lipidemic sesquiterp enes and new sesquiterp ene glycosides from the leaves of artichoke (Cynara scolymus L. ) : structure require- ment and mode of action[J] . Bioorganic & Medicinal Chemistry Letters , 2003 , 13(2) : 223-228.
[10] BUNDY R , WALKER A F , MIDDLETON R W , et al. Arti- choke leaf extract ( Cynara scolymus ) reduces plasma cholesterol in otherwise healthy hyp ercholesterolemic adults : A randomized , double blind placebo controlled trial[J] . Phytomedicine , 2008 , 15(9) : 668-675.
[11] WALKER A F , MIDDLETON R W , PETROWICZ O . Arti- choke leaf extract reduces sym ptoms of irritable bowel syndrome in a post-marketing surveillance study[J] . Phytother- apy Research , 2001 , 15(1) : 58-61.
[12] MISAEL P C E , DE GUADALUPE T , DEL CSGM P , et al. Effect of Cynara scolymus (artichoke) in homeopathic doses on body mass index in obese and overweight patients[J] . Biomed Pharmacol J , 2014 , 7 : 525-533.
[13] RONDANELLI M , OPIZZI A , FALIVA M , et al. Metabolic management in overweight subj ects with naive im paired fasting glycaemia by means of a highly standardized extract from cynara scolymus:a double-blind,placebo-controlled , randomized clinical trial[J] . Phytotherapy Research , 2014 , 28 (1) : 33-41.
[14] HOLTMANN G , ADAM B , HAAG S , et al. Efficacy of arti- choke leaf extract in the treatment of patients with functional dyspepsia : a six-week placebo-controlled , double-blind , multi- centre trial [J] . Alimentary Pharmacology & Therap eutics , 2003 , 18(11/12) : 1 099-1 105.
[15] KIRCHHOFF R , BECKERS C H , KIRCHHOFF G M , et al. Increase in choleresis b y means of artichoke extract [J] . Phyto- medicine , 1994 , 1(2) : 107-115.
[16] ROMANI A , PINELLI P , CANTINI C , et al. Characterization of Violetto di Toscana , a typical Italian variety of artichoke (Cynara scolymus L. ) [J] . Food Chemistry , 2006 , 95 (2) : 221-225.
[17] DRANIK L I. Phenol compounds from some plants of the Com- positae family : Artichoke (Cynara scolymus ) [J] . Fenol'nye Soedineniya i Ikh Biologicheskie Funktsii , Materialy Vse- soyuznogo Simpoziuma po Fenol'nym Soedineniyam , 1968 , 12 : 53-60.
[18] MULINACCI N , PRUCHER D , PERUZZI M , et al. Commercial and laboratory extracts from artichoke leaves : estimation of caffeoyl esters and flavonoidic compounds content [J] . Journal of Pharmaceutical and Biomedical Analysis ,2004 , 34 ( 2 ) : 349-357.
[19] ORLOVSKAYA T V , LUNEVA I L , CHELOMBIT KO V A. Chemical composition of Cynara scolymus leaves[J] . Chemistry of Natural Compounds , 2007 , 43(2) : 239-240.
[20] SARAWEK S , FEISTEL B , PISCHEL I , et al. Flavonoids of Cynara scolymus possess potent xanthinoxidase inhibitory ac- tivity in vitro but are devoid of hypouricemic effects in rats after oral application[J] . Planta Medica , 2008 , 74(3) : 221-227.
[21] GEBHARDT R. Prevention of taurolithocholate-induced hepatic bile canalicular distortions b y HPLC-characterized extracts of artichoke (Cynara scolymus) leaves[J] . Planta Medica , 2002 , 68(9) : 776-779.
[22] SCHTZ K , KAMMERER D , CARLE R , et al. Identification and quantification of caffeoylquinic acids and flavonoids from ar- tichoke ( Cynara scolymus L. ) heads , juice , and pomace b y HPLC-DAD-ESI/MSn [J] . Journal of Agricultural and Food Chemistry , 2004 , 52(13) : 4 090-4 096.
[23] EL-NEGOUMY S I , EL-SAYED N H , SALEH N A M. Fla- vonoid glycosides of Cynara scolymus[J] . Fitoterapia , 1987 , 58 (3) : 178-180.
[24] HINOU J , HARVALA C , PHILIANOS S. Polyphenolic sub- stances of Cynara scolymus L. leaves[J] . Annales Pharmaceu- tiques Francaises , 1989 , 47(2) : 95-98.
[25] LATTANZIO V , VAN SUMERE C F. Changes in phenolic compounds during the development and cold storage of artichoke (Cynara scolymus L. ) heads[J] . Food Chemistry , 1987 , 24(1) : 37-50.
[26] AUBERT S , FOURY C. Couleur et pigmentation anthocyani- que de l'artichaut (Cynara scolymus L. )[J] . Studi Sul Carciofo , 1981 , 31 : 57-76.
[27] SCHTZ K , PERSIKE M , CARLE R , et al. Characterization and quantification of anthocyanins in selected artichoke (Cynara scolymus L. ) cultivars b y HPLC-DAD-ESI-MSn [J] . Analytical and Bioanalytical Chemistry , 2006 , 384(7/8) : 1 511-1 517.
[28] Zhu N, Peng P, Zhao L, et al. Research progress on common caffeoylquinic acid compounds in plants [C]// 8th Annual Conference of the Chinese Society of Traditional Chinese Medicine Chemistry Branch. Beijing: Chinese Society of Traditional Chinese Medicine Chemistry Branch, 2013: 192-201.
[29] PANIZZI L , SCARPATI M L. Constitution of cynarine , the active p rinciple of the artichoke[J] . Nature , 1954 , 174 : 1 062.
[30] ZHU Xian-feng , ZHANG Hong-xun , LO R. Phenolic com- pounds from the leaf extract of artichoke (Cynara scolymus L. ) and their antimicrobial activities[J] . Journal of Agricultural and Food Chemistry , 2004 , 52(24) : 7 272-7 278.
[31] LATTANZIO V , CARDINALI A , DI VENERE D , et al. Browning phenomena in stored artichoke (Cynara scolymus L. ) heads : enzymic or chemical reactions ? [J] . Food Chemistry , 1994 , 50(1) : 1-7.
[32] AZZINI E , BUGIANESI R , ROMANO F , et al. Absorption and metabolism of bioactive molecules after oral consumption of cooked edible heads of Cynara scolymus L. ( cultivar Violetto di Provenza) in human subj ects : a pilot study[J] . British Journal of Nutrition , 2007 , 97(5) : 963-969.
[33] GIL-IZQUIERDO A , GIL M I , CONESA M A , et al. The effect of storage temperatures on vitamin C and phenolics content of artichoke (Cynara scolymus L. ) heads[J] . Innovative Food Science & Emerging Technologies , 2001 , 2(3) : 199-202.
[34] VIGH S , CZIAKY Z , SINKA L T , et al. Comparative chemo- mapping of phytoconstituents from different extracts of globe artichoke-Cynara scolymus L. [J] . Studia Universitatis Babes- Bolyai , Chemia , 2017 , 62(2) : 125-143.
[35] Song Shuhui, He Hongju, Tang Xiaowei, et al. Research on the extraction of polyphenolic compounds from artichoke using ultrasonic technology [J]. Anhui Agricultural Science, 2007, 35(30): 9694-9695.
[36] Zhang Jun, Shao Min, Chen Jianbing, et al. Study on the process conditions of microwave-assisted extraction of polyphenols from artichoke [J]. Food Industry Science and Technology, 2008(11): 173-175.
[37] Zhao Youyi, Wang Qizhi, Zhang Jianhua, et al. Optimization of the extraction process of total phenolic acids from artichoke by orthogonal test method [J]. Food Research and Development, 2013, 34(20): 15-17.
[38] Yang Liu. Research on the analysis and determination of polyphenols in tea [D]. Taiyuan: Shanxi University, 2005: 22-23.
[39] Xue Aihui, Cui Meng, Zou Huajie, et al. Electro-spray extraction ionization mass spectrometry analysis of polyphenolic compounds in Osmanthus fragrans[J]. Food Science, 2018, 39(16): 221-226.
[40] Feng Li, Song Shuhui. Determination of polyphenol content in artichoke leaves by Folin-Ciocalteu colorimetric method [J]. Chinese Food and Nutrition, 2011, 17(1): 62-64.
[41] FRATIANNI F , TUCCI M , De PALMA M , et al. Polyphenolic composition in different parts of some cultivars of globe artichoke (Cynara cardunculus L. var. scolymus (L. ) Fio- ri)[J] . Food Chemistry , 2007 , 104(3) : 1 282-1 286.
[42] REZAZADEH A , GHASEMNEZHAD A , BARANI M , et al. Effect of salinity on phenolic composition and antioxidant activity of artichoke (Cynara scolymus L. ) leaves[J] . Research Journal of Medicinal Plant , 2012 , 6(3) : 245-252.
[43] Liang Lifang. Research on high performance liquid chromatography detection technology of flavonoids and antibiotics [D]. Chongqing: Southwest University, 2008: 21.
[44] Cao Peiqin, Huang Jian'an, Li Yinhua, et al. Simultaneous determination of chlorogenic acid and artichokein in artichoke leaves by high performance liquid chromatography [J]. Hunan Agricultural Science, 2014(7) : 8-10.
[45] Zhang Jun, Du Gang, Tian Menghua, et al. Determination of chlorogenic acid content in artichoke leaves by UPLC [J] . Northern Horticulture, 2011(20) : 46-48.
[46] Luo Kui, Wei Yangji, Yang Lili, et al. Study on the content of polyphenols in processed artichoke products [J]. Chinese Agricultural Science Bulletin, 2012, 28(33): 273-279.
[47] PANDINO G , LOMBARDO S , MAUROMICALE G , et al. Profile of polyphenols and phenolic acids in bracts and recepta- cles of globe artichoke ( Cynara cardunculus var. scolymus ) germ plasm[J] . Journal of Food Composition and Analysis , 2011 , 24(2) : 148-153.
[48] TROUILLAS P , CALLISTE C , ALLAIS D , et al. Antioxidant , anti-inflammatory and antip roliferative properties of sixteen water plant extracts used in the Limousin countryside as herbal teas [J] . Food Chemistry , 2003 , 80(3) : 399-407.
[49] Song Shuhui, Zhang Limei, Bao Shanfen, et al. In vitro antioxidant activity of artichoke leaf extract [J]. Food Research and Development, 2011, 32(5): 41-45.
[50] Yang Haiying, Li Jinyin, Wang Xuemei, et al. Study on the antioxidant properties of artichoke extract [J]. Anhui Agricultural Science, 2008, 36(20): 8641-8642.
[51] EVCKOVA P , GLATZ Z , SLANINA J . Analysis of artichoke (Cynara cardunculus L. ) extract by means of micellar electrokinetic capillary chromatography [J] . Electrophoresis , 2002 , 23(2) : 249-252.
[52] JUN Neung-j ae , JANG Ki-chang , KIM Seong-cheol , et al. Radical scavenging activity and content of cynarin (1 , 3-dicaf- feoylquinic acid) in Artichoke ( Cynara scolymus L. ) [J] . Journal of Applied Biological Chemistry , 2007 , 50 ( 4 ) : 244-248.
[53] PEREZ-GARCA F , ADZET T , CANIGUERAL S. Activity of artichoke leaf extract on reactive oxygen species in human leu- kocytes[J] . Free Radical Research , 2000 , 33(5) : 661-665.
[54] VAMANU E , VAMANU A , NITA S , et al. Antioxidant and antimicrobial activities of ethanol extracts of Cynara scolymus (Cynarae folium , Asteraceae family) [J] . Tropical Journal of Pharmaceutical Research , 2011 , 10(6) : 777-783.
[55] Yang Kexia. Chemical composition and activity research of artichoke [D]. Kunming: Kunming University of Science and Technology, 2015: 45-46.
[56] GEBHARDT R , FAUSEL M. Antioxidant and hepatoprotec- tive effects of artichoke extracts and constituents in cultured rat hepatocytes[J] . Toxicology in Vitro , 1997 , 11(5) : 669-672.
[57] Yao Min, Li Xingbin, Zheng Jun. Phytomedicines for hyperlipidemia [J]. Foreign Medicine: Phytomedicine Supplement, 2003, 18(3): 107-109.
[58] Song Shuhui, Zhao Lin, Wang Wenqi, et al. Study on the anti-hyperlipidemic effect of artichoke leaf extract [J]. Food Science and Technology, 2010, 35(12): 194-197.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






