Study on Echinacea Purpurea Extract for Immune
Echinacea purpurea (Linn.) Moench is a perennial herb in the Asteraceae family, also known as echinacea. Echinacea purpurea is rich in chemical components and has a variety of pharmacological activities. It is a much-discussed immunomodulator. At present, the academic community has made some progress in research on the chemical composition, pharmacological effects and quality control of Echinacea purpurea, but there is a lack of systematic reports on the subject. This article therefore provides a review of the chemical composition, pharmacological effects and quality control of Echinacea purpurea, with a view to laying a foundation for the rational development and application of Echinacea purpurea.
1 Active ingredients in Echinacea purpurea extract
According to reports in domestic and foreign literature, Echinacea purpurea extract mainly contains caffeic acid derivatives, alkylamides, essential oils, polysaccharides and other ingredients.
1.1 Caffeic acid derivatives
Caffeic acid derivatives are the most reported chemical components in Echinacea purpurea and are also its main active ingredients. Cichoric acid, monocaffeoyl tartaric acid, caffeic acid, chlorogenic acid and echinacoside have been isolated from Echinacea purpurea [1]. Among these, cichoric acid and caffeic acid are its main active ingredients. The components of the caffeic acid derivatives in echinacea purpurea are shown in Table 1 [1-8].
1.2 Alkylamides
Research on alkylamide components was initially aimed at finding an insecticide from Echinacea purpurea, but it was later found to have the effect of enhancing immunity. Most of the alkylamide components in Echinacea purpurea are unsaturated fatty amides, which are mainly found in the roots [9]. The alkylamide components in Echinacea purpurea are shown in Table 2 [4, 7, 10-15].

1.3 Volatile oils
The components of the Echinacea essential oil play an important role in anti-inflammatory and other aspects. Xue Yafeng [16] was the first to use solid-phase microextraction combined with gas chromatography-mass spectrometry (GC-MS) to identify compounds such as α-pinene from Echinacea. Subsequently, a variety of volatile oil components were successively identified and separated. The components of the Echinacea essential oil are shown in Table 3 [17-20].
1.4 Polysaccharides and glycoproteins
Two types of polysaccharides with immunostimulatory properties can be isolated from the aqueous extract of the aerial parts of Echinacea purpurea: 4-methoxy-glucurono-arabino-xylan and acidic arabinose-rhamnose-galactan. It has been reported in the literature that polysaccharides, inulin-type components and acidic highly branched arabinogalactan polysaccharides can be obtained from the aerial parts of Echinacea by the juicing method [21]. Meanwhile, Classen et al. [22] isolated an arabinogalactan protein (AGP) from the pressed juice of the aerial parts of Echinacea, and by binding and 13C nuclear magnetic resonance analysis showed that AGP is composed of a highly branched core polysaccharide composed of 3-, 6- and 3,6-linked galactose residues, with arabinose and glucuronic acid units at the ends. In addition, Li et al. [23] used diethylaminoethyl (DEAE) ion exchange and gel filtration chromatography to isolate three polysaccharide components (EPPS-1, EPPS-2 and EPPS-3) from Echinacea purpurea. Analysis of the methylation of EPPS-3 revealed that EPPS-3 has seven bond types, the main forms being 1,4-glucopyranose and 1,4-galactopyranose, and compared with the other two sugars, EPPS-3 has anti-inflammatory effects.
1.5 Other ingredients
In addition to the above ingredients, Echinacea purpurea extract also contains flavonoids, steroidal components and various inorganic elements [24].
2 Pharmacological effects
2.1 Immune regulation
Echinacea purpurea extract has an immunomodulatory effect, which is mainly achieved by enhancing T cell activity, stimulating the killing of P815 tumor cells by macrophages, increasing the peripheral blood CD4+/CD8+ content, and enhancing antigen specificity [25-27]. Zhong Yingjie et al. [28] found that Echinacea purpurea can promote the production of hemolytic antibodies and enhance the activity of natural killer (NK) cells, as well as enhance humoral immunity, cellular immunity and non-specific immunity. At the same time, Echinacea purpurea can promote the proliferation of peripheral blood mononuclear cells, thereby exerting an immunomodulatory effect [29]. Ijzerman et al. [30] established an immunosuppression model in mice by intraperitoneally injecting cyclophosphamide, and after gavage with Echinacea purpurea extract, the expression of interleukin-2 (IL-2), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-alpha) in the mouse serum increased, concluding that Echinacea purpurea extract can increase the secretion levels of IL-2, IL-6 and TNF-α in mice and improve their immunity. In addition, the high levels of complex polysaccharides present in the aqueous extract of Echinacea purpurea root can activate mouse dendritic cells and enhance their immune function [31].
2.2 Anti-inflammatory
Echinacea purpurea ethanol extract has an anti-inflammatory effect on ear swelling caused by xylene and on foot swelling caused by egg white in mice within the experimental dose range, and the anti-inflammatory activity increases with increasing extract dose [32]. It has been reported in the literature that alkylamides can inhibit the production of cytokines, chemokines and prostaglandins, and treat allergic and mast cell-mediated inflammatory responses [33]. Xu Tianli et al. [34] also found that Echinacea polysaccharides have a significant inhibitory effect on xylene-induced ear swelling in mice. It also inhibits the increased expression of TNF- α and IL- 6 in mouse serum caused by endotoxin damage, and reduces the relative expression levels of inflammatory factors such as cyclooxygenase (COX)- 2 and inducible nitric oxide synthase (iNOS) in lung tissue [35].
Li et al. [23] found that Echinacea polysaccharide EPPS-3 can effectively counteract the effects of lipopolysaccharide (LPS) and significantly reduce lung damage by establishing a mouse sepsis model induced by LPS. Fast et al. [36] showed that Echinacea polysaccharide inhibits the production of TNF-α stimulated by Toll-like receptor (TLR) 1/2 through the PI3K/Akt signaling pathway. It has also been reported in the literature that the volatile oil components of Echinacea purpurea have an inhibitory effect on different animal models of inflammation, such as ear swelling in mice caused by xylene, foot swelling in rats caused by egg white, and proliferative inflammation of granuloma tissue in mice caused by cotton balls. Its effect is achieved by inhibiting the expression of inflammatory cytokines such as IL-2, IL-6, and TNF-α in the blood [37]. In addition, in terms of relieving skin pain, Echinacea can relieve the symptoms of atopic dermatitis by exerting its significant anti-inflammatory and restoring the lipid barrier of the epidermis [38]. Schapowal et al. [39] found that Echinacea can reduce the risk of recurrent respiratory tract infections. Due to the different preparation methods, the efficacy may differ. Immunocompromised and susceptible people benefit the most. While reducing the risk of infection, prevent the occurrence of complications such as pneumonia, otitis media, and tonsillitis.
2.3 Antioxidant and free radical scavenging
The lutein in Echinacea has strong antioxidant activity in vitro can remove reactive oxygen species (ROS) produced by oxidative stress and increase the total cellular glutathione (GSH) level of oxidative stress [40]. Modarai et al. [41] found that the alkylamide components in Echinacea purpurea can inhibit cytochrome P4503A4 (CYP3A4). It has also been reported in the literature that phenolic acids such as chicoric acid and caffeic acid have strong antioxidant properties and the ability to scavenge free radicals in living organisms. The 1,1-diphenyl-2-trinitrobenzene hydrazone (DPPH) free radical scavenging capacity increases in a dose-dependent manner with the concentration of phenolic acids, and the DPPH free radical scavenging capacity depends on the number of hydroxyl groups and the position of the substituents in the chicoric acid. In particular, the phenolic ring with the two adjacent hydroxyl groups has a stronger ability to scavenge free radicals [42]. In addition, chicoric acid also alleviates methotrexate (MTX)-induced oxidative damage by activating the Nrf2/ARE/HO-1 signaling pathway [43].
Some researchers have found that the active ingredients in Echinacea purpurea can be used as electron or hydrogen proton donors to directly eliminate or indirectly inhibit free radicals, thereby preventing a series of free radical reactions. This may be the mechanism of action of phenolic acids in terms of oxidation resistance [44]. In addition, Echinacea polysaccharides can activate the NF-E2-related factor 2 (Nrf2) signaling pathway, inhibit apoptosis, and exert an antioxidant effect [45].
2.4 Antiviral
Echinacea can exert an antiviral effect by regulating the function of macrophages, and can also change the degree of macrophage antiviral effect [46]. It has been reported in the literature that Echinacea has varying degrees of inhibitory effects on cell damage caused by influenza virus, respiratory syncytial virus (RSV), Coxsackievirus B (CVB) and herpes simplex virus type 2 (HSV-2) [24]. and also has an indirect antiviral effect by stimulating the production of interferon-alpha (IFN-α) and interferon-beta (IFN-β). Pleschka et al. [47] found that Echinacea inhibited the binding activity of the H5N1 highly pathogenic avian influenza virus (HPAIV) to receptors, interfering with the virus entering cells. Echinacea also inhibits the secretion of inflammatory cytokines such as IL-6 and IL-8, inhibits RSV penetration, and has strong antiviral activity against membrane viruses [48]. Echinacoside also inhibits human immunodeficiency virus (HIV) integration by non-competitively but reversibly inhibiting the virus cD-NA from entering the host chromosome, preventing HIV infection [49]. In addition, TNF is produced by immune cells to resist viruses, and has a direct antiviral effect. It also induces the expression of other antiviral cytokines. Some studies have found that Echinacea can increase the level of TNF cytokine produced by ConA-activated splenocytes, enhance the production of TNF, and promote antiviral immune responses [24].
2.5 Effect on the respiratory system
Echinacea has a therapeutic effect on respiratory diseases associated with asthma. Capek et al. [50] found through pharmacological experiments that the polysaccharide-phenolic protein complex obtained by extracting and isolating echinacea after soaking in alkali solution has a significant bronchodilator and antitussive effect. Echinacea's bronchodilatory effect is significantly better than that of the clinically used asthma drug salbutamol, and its antitussive effect is similar to that of codeine [51]. Vimalanathan et al. [52] found that echinacea can significantly reduce the expression of intercellular adhesion molecule-1 (ICAM-1), fibronectin and platelet-activating factor receptor (PAFr) expression, thereby reducing the adhesion of Haemophilus influenzae (NTHi) and Staphylococcus aureus. It also inhibits the production of inflammatory cytokines by blocking the expression of nuclear factor κB (NF-κB) and TLR-4 protein, thereby reducing the risk of respiratory complications.
2.6 Other effects
The alkylamide components in Echinacea purpurea are lipophilic compounds with anti-inflammatory activity. They exhibit antifungal properties by inhibiting the activity of cyclooxygenase and lipoxygenase [53]. The polysaccharide components produce a hyaluronic acid-polysaccharide complex in the body, which inhibits hyaluronidase, promotes fibroblast growth, and is beneficial for wound healing [54]. In addition, Tsai et al. [55] found that chicoric acid inhibits the growth of human colon cancer cells (HCT-116) by inhibiting telomerase activity and the expression of b-cyclin, thereby inducing apoptosis characterized by caspase-9 activation and PARP cleavage, and exerting an anti-cancer effect.
3 Quality control
Quality control is the main means of ensuring the safety and efficacy of Echinacea in clinical use. Only research on ingredients and pharmacological effects carried out on the basis of guaranteed quality is meaningful. At present, research on the quality control of Echinacea is not comprehensive enough, and it is difficult to formulate unified standards using a combination of methods. The next step of research could consider a comprehensive exploration using modern analytical methods and techniques.
3.1 Identification of characteristics
The United States Pharmacopoeia (USP) provides a detailed description of the characteristics of the roots, stems, flowers and leaves of Echinacea purpurea. Han Linna et al. [56] studied the morphological characteristics of Echinacea purpurea and concluded that the quality of the herb can be preliminarily judged by phenotypic traits such as plant height, peduncle height, and the height-to-width ratio of the peduncle. The content of the main ingredients in the herb is negatively correlated with the altitude, so Echinacea purpurea is not suitable for cultivation at high altitudes. Sun Junying [57] and Zhong Yingjie et al. [28] identified the morphological, microscopic and physical and chemical characteristics of Echinacea purpurea, providing identification criteria for the morphological and tissue characteristics for the formulation of quality standards for this medicinal herb. The specific identification characteristics of Echinacea purpurea are shown in Table 4.
3.2 Microscopic identification
Some scholars have carried out microscopic identification of the transverse sections and powder of the roots and leaves of Echinacea purpurea [58-59], which provides a basis for the quality control of Echinacea purpurea. The specific microscopic identification characteristics are shown in Table 5.
3.3 Component determination
Wang Zhibin et al. [20] used GC-MS to detect 47 components in the essential oil of Echinacea purpurea and identify 22 compounds. The percentage content of each component was calculated using the area normalization method. Luo Xubiao et al. [60] used high performance liquid chromatography (HPLC) for the first time, using ammonium acetate-methanol as the mobile phase, a C18 reverse-phase column and a diode array detector to quickly and accurately analyze the content of the active ingredient chicoric acid in Echinacea compound preparations, laying the foundation for the quality control of Echinacea. At the same time, some scholars used high performance liquid chromatography-electrospray mass spectrometry to analyze and identify the content of chicoric acid and caffeic acid as well as 11 amide components in Echinacea purpurea extract [4]. This method is efficient and the measurement results are accurate. In addition, HPLC can be used to distinguish Echinacea purpurea from different origins in addition to evaluating the consistency of Echinacea purpurea medicinal materials.
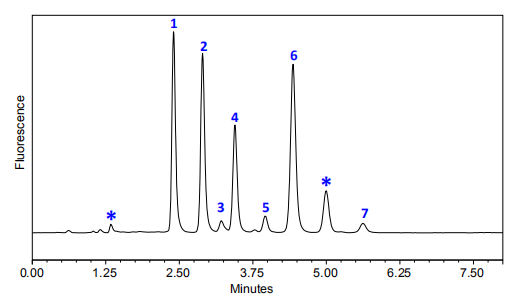
Sun Liping et al. [3] used HPLC to quantitatively analyze seven phenolic acid components in Echinacea extracts from different origins: 3,4-dihydroxybenzoic acid, chlorogenic acid, caffeic acid, cinnamic acid, p-coumaric acid, ferulic acid, and chicoric acid. The results showed that the total phenolic acid content and the content of the main index component, chicoric acid, varied greatly depending on the origin. Traditional Chinese medicine has the characteristics of multiple components and multiple targets. It is difficult to accurately evaluate the quality of medicinal materials by a single component. Traditional Chinese medicine fingerprints can simultaneously determine multiple components and have been used in recent years as one of the means to evaluate the quality of Echinacea purpurea medicinal materials. Kong Lingfeng [61] used HPLC to investigate the fingerprints of 10 batches of Echinacea purpurea herbs and established a common model. He found 11 common peaks and a good similarity between the fingerprints of the samples to be tested and the common model, which can be used as one of the methods for evaluating the quality of Echinacea purpurea herbs.
4 Conclusion
Echinacea extract is rich in chemical components and has various pharmacological activities, such as immunomodulation, anti-inflammation, anti-oxidation, free radical scavenging, antiviral and antibacterial activities. It is especially valued internationally as an immunomodulator. At present, research on the chemical composition, pharmacological effects and quality control of Echinacea has made some progress. However, due to the complex active ingredients of Echinacea, research on Echinacea is not yet comprehensive and in-depth. For example, quality control is still weak, with a single index component, and the content of echinacoside is mostly used as an indicator to evaluate the quality of Echinacea. In terms of chemical composition, the research on effective ingredients is too one-sided. researchers have done more research on caffeic acid derivatives, and other types of ingredients have yet to be explored in depth; in terms of pharmacological effects, the research on the pharmacological activity of Echinacea purpurea is not comprehensive enough, focusing mainly on immune regulation, and the pharmacological basis and mechanism of action of other pharmacological effects have yet to be further elucidated.

In view of the above problems, the following points should be addressed in future research on Echinacea: (1) in terms of quality control, DNA molecular genetic marker technology and DNA fingerprint technology can be used to provide accurate digital identification information for the identification of medicinal materials; (2) in terms of chemical composition, a comprehensive study of various active ingredients is required, as well as a clear understanding of their pharmacological mechanisms. It is also possible to carry out structural modification of compounds to solve problems such as poor absorption and difficult to utilize, etc.; ③ in terms of pharmacological effects, the pharmacological active sites and mechanisms of action of the active ingredients should be fully studied, and their mechanisms of action can be elucidated at the cellular and molecular levels. In addition, the active ingredients of Echinacea, such as echinacoside, have low oral bioavailability and poor stability in the stomach. Formulations such as sprays or aerosols can be considered to promote the absorption of the active ingredients.
With people's living standards and health awareness constantly increasing, the application prospects of echinacea are becoming more and more promising. It can be studied to be made into new pharmaceutical preparations with immune enhancement and other effects for clinical use, and it can also be promoted as a health product for daily consumption to maximize its medicinal value.
Reference:
[1] NiSSLEIN B,KURZMANN M,BAUER R,et al. Enzymatic degradation of cichoric acid in Echinacea purpurea prepa- rations[J]. J Nat Prod,2000,63(12):1615- 1618.
[2] WANG X,GENG Y,LI F,et al. Preparative separation of cichoric acid from Echinacea purpurea by pH-zone- refining counter-current chromatography[J]. J Chromatogr A,2006,1103(1):166- 169.
[3] Sun Liping, Qi Haiyan, Zheng Hongwei, et al. Determination of the content of seven phenolic acids in Echinacea purpurea by high performance liquid chromatography and cluster analysis [J]. Pharmaceutical Guide, 2020, 39 (6): 831-835.
[4] CECH N B,ELEAZER M S,SHOFFNER L T,et al. High performance liquid chromatography/electrospray ionization mass spectrometry for simultaneous analysis of alkamides and caffeic acid derivatives from Echinacea purpurea extracts[J]. J Chromatogr A,2006,1103(2):219- 228.
[5] PELLATI F,BENVENUTI S,MAGRO L,et al. Analysis of phenolic compounds and radical scavenging activity of Echinacea spp[J]. J Pharm Biomed Anal,2004,35(2): 289- 301.
[6] POMPONIO R,GOTTI R,HUDAIB M,et al. Analysis of phenolic acids by micellar electrokinetic chromatography: application to Echinacea purpurea plant extracts[J]. J Chromatogr A,2002,945(1- 2):239- 247.
[7] LUO X B,CHEN B,YAO S Z,et al. Simultaneous anal- ysis of caffeic acid derivatives and alkamides in roots and extracts of Echinacea purpurea by high-performance liquid chromatography-photodiode array detection-electrospray mass spectrometry[J]. J Chromatogr A,2003 ,986(1):73- 81.
[8] Chen Qiuling, Wang Lei, Feng Feng. Study on the chemical composition of the aboveground part of Echinacea purpurea [J]. Chinese Materia Medica, 2013, 36(5): 739- 743.
[9] Zhang Ying, Liu Ke, Wu Lijun. Research progress on the medicinal plants of the Echinacea genus [J]. Chinese Herbal Medicine, 2001, 32(9): 852-855.
[10] CHEN Y ,FU T ,TAO T ,et al. Macrophage activating effects of new alkamides from the roots of Echinacea species[J]. J Nat Prod,2005,68(5):773- 776.
[11] MO/LGAARD P,JOHNSEN S,CHRISTENSEN P ,et al. HPLC method validated for the simultaneous analysis of cichoric acid and alkamides in Echinacea purpurea- Plants and products[J]. J Agric Food Chem,2003,51(24): 6922- 6933.
[12] HOHMANN J ,RéDEI D ,FORGO P ,et al. Alkamides and a neolignan from Echinacea purpurea roots and the interaction of alkamides with G-protein-coupled cannabi- noid receptors[J ]. Phytochemistry ,2011 ,72( 14- 15 ): 1848- 1853.
[13] HOHMANN J,RéDEI D,FORGO M G,et al. GC- MS analysis of the lipophilic principles of Echinacea pur- purea and evaluation of cucumber mosaic Cucumovirus infection[J]. J Pharm Biomed Anal,2002,29(6):1053- 1060.
[14] BINNS S E,LIVESEY J F,ARNASON J T,et al. Phy- tochemical variation in Echinacea from roots and flow- erheads of wild and cultivated populations[J]. J Agric Food Chem,2002,50(13):3673- 3687.
[15] CLIFFORD LJ,NAIR MG,RANA J,et al. Bioactivity of alkamides isolated from Echinacea purpurea(L.)Moench[J]. Phytomedicine,2002,9(3):249- 253.
[16] Xue Yafeng. Study on the chemical composition of the aboveground part of Echinacea purpurea [D]. Yangling: Northwest A&F University, 2008.
[17] MAZZA G,COTTRELL T. Volatile components of roots, stems ,leaves ,and flowers of Echinacea species[J]. J Agric Food Chem,1999,47(8):3081- 3085.
[18] NYALAMBISA M,OYEMITAN I A,MATEWU R,et al. Volatile constituents and biological activities of the leaf and root of Echinacea species from South Africa[J]. Saudi Pharm J,2017,25(3):381- 386.
[19] Huang Huaxi, Zang Qingmin, Yi Xiuhong, et al. Analysis of the volatile components of Echinacea purpurea from different parts [J]. Northern Horticulture, 2020 (17): 102-109.
[20] Wang Zhibin, Song Mengmeng, Liu Hua, et al. Analysis of the chemical composition of the volatile oil of the aboveground parts of Echinacea purpurea by GC-MS [J]. Chemical Engineer, 2017, 31(7): 19-22.
[21] BARNES J,ANDERSON L A,GIBBONS S,et al. Echi- nacea species(Echinacea angustifolia(DC. ) Hell.,Echi- nacea pallida( Nutt . ) Nutt . ,Echinacea purpurea( L . ) Moench):a review of their chemistry,pharmacology and clinical properties[J]. J Pharm Pharmacol,2005,57(8): 929- 954.
[22] CLASSEN B,WITTHOHN K,BLASCHEK W. Charac- terization of an Arabinogalactan-protein isolated from pressed juice of Echinacea purpurea by precipitation with the beta-glucosyl Yariv reagent[J]. Carbohydr Res, 2000,327(4):497- 504.
[23] LI Q ,YANG F F ,HOU R R ,et al. Post-screening characterization of an acidic polysaccharide from Echi- nacea purpurea with potent anti-inflammatory properties in vivo[J]. Food Funct,2020,11(9):7576- 7583.
[24] Liu Yichen. Study on phenolic acid components in Echinacea purpurea introduced in China [D]. Changsha: Hunan Normal University, 2008.
[25] Xiao Peigen. Echinacea purpurea and its preparations, an internationally popular immunomodulator [J]. Chinese Herbal Medicine, 1996, 27(1): 46-48.
[26] FONSECA F N ,PAPANICOLAOU G ,LIN H ,et al . Echinacea purpurea(L.)Moench modulates human T-cell cytokine response[J]. Int Immunopharmacol,2014,19(1): 94- 102.
[27] Ni Yaodi, Xu Li, Du Jian. Effect of Echinacea polysaccharide on T cell subsets in peripheral blood of IBDV-vaccinated immune chicks [J]. Chinese Journal of Veterinary Medicine, 2014, 50(5): 57-59.
[28] Zhong YJ, Jiang TZ, Fu H, et al. Pharmacological studies on Echinacea purpurea raw herbs collected at different times of year.
[29] Wu Hua, Nardone A, Lacetera N. Effect of Echinacea purpurea extract PolinaceaTM on the immune function of peripheral blood mononuclear cells from dairy cows [J]. Chinese Journal of Veterinary Science, 2010, 40(5): 532-536.
[30] Yijianing, Fu Jian, Fu Lirong, et al. Effect of Echinacea purpurea extract on serum cytokines in mice [J]. Chinese Journal of Veterinary Medicine, 2016, 35(3): 34-36.
[31] BENSON J M,POKORNY A J,RHULE A,et al. Echi- nacea purpurea extracts modulate murine dendritic cell fate and function[J]. Food Chem Toxicol,2010,48(5): 1170- 1177.
[32] Cheng Yongxue, Liu Yongfang, Sun Qingxin, et al. Extraction process and anti-inflammatory activity of Echinacea purpurea flower active ingredients [J]. Pharmaceutical Research, 2018, 37(3): 139-141, 145.
[33] GULLEDGE T V,COLLETTE N M,MACKEY E,et al. Mast cell degranulation and calcium influx are inhibited by an Echinacea purpurea extract and the alkylamide dodeca- 2E,4E-dienoic acid isobutylamide[J]. J Ethno- pharmacol,2018,212:166- 174.
[34] Xu Tianli, Hou Rannan, Li Qiu, et al. Preparation of Echinacea purpurea purified polysaccharide and its anti-inflammatory effect [J]. Heilongjiang Animal Husbandry and Veterinary Medicine, 2019 (22): 133-136.
[35] Li Yanyun. Research on the mechanism of action of Echinacea polysaccharides on endotoxin in Escherichia coli [D]. Qinhuangdao: Hebei University of Science and Technology, 2015.
[36] FAST D J,BALLES J A,SCHOLTEN J D,et al. Echi- nacea purpurea root extract inhibits TNF release in response to Pam3Csk4 in a phosphatidylinositol- 3-kinase dependent manner[J]. Cell Immunol,2015,297(2):94- 99.
[37] YU D Q,YUAN Y,JIANG L,et al. Anti-inflammatory effects of essential oil in Echinacea purpurea L[J]. Pak J Pharm Sci,2013,26(2):403- 408.
[38] OLáH A,SZAB6- PAPP J,SOEBERDT M,et al. Echinacea purpurea-derived alkylamides exhibit potent anti-inflam- matory effects and alleviate clinical symptoms of atopic eczema[J]. J Dermatol Sci,2017,88(1):67- 77.
[39] SCHAPOWAL A,KLEIN P,JOHNSTON S L. Echinacea reduces the risk of recurrent respiratory tract infections and complications:a meta-analysis of randomized con- trolled trials[J]. Adv Ther,2015,32(3):187- 200.
[40] KARG C A,WANG P,VOLLMAR A M,et al. Re-opening the stage for Echinacea research- Characterization of phylloxanthobilins as a novel anti-oxidative compound class in Echinacea purpurea[J]. Phytomedicine,2019, 60:152969.
[41] MODARAI M,YANG M,SUTER A,et al. Metabolomic profiling of liquid Echinacea medicinal products with in vitro inhibitory effects on cytochrome P450 3A4 (CYP3A4)[J]. Planta Med,2010,76(4):378- 385.
[42] CHIOU S Y,SUNG J M,HUANG P W,et al. Antioxidant, antidiabetic,and antihypertensive properties of Echinacea purpurea flower extract and caffeic acid derivatives using in vitro models[J]. J Med Food,2017,20(2):171- 179.
[43] HUSSEIN O E,HOZAYEN W G,BIN-JUMAH M N, et al. Chicoric acid prevents methotrexate hepatotoxicity via attenuation of oxidative stress and inflammation and up-regulation of PPARγ and Nrf2/HO- 1 signaling[J]. Environ Sci Pollut Res Int,2020,27(17):20725- 20735.
[44] Zhang Jing, Li Zhiying, Cheng Fei, et al. Research progress on the molecular mechanism of the antioxidant effect of Echinacea purpurea. Contemporary Animal Husbandry, 2016 (36): 44-46.
[45] HOU R,XU T,LI Q,et al. Polysaccharide from Echi- nacea purpurea reduce the oxidant stress in vitro and in vivo[J]. Int J Biol Macromol,2020,149:41- 50.
[46] SENCHINA D S,MARTIN A E,BUSS J E. Effects of Echinacea extracts on macrophage antiviral activities[J]. Phytother Res,2010,24(6):810- 816.
[47] PLESCHKA S,STEIN M,SCHOOP R,et al. Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus(H5N1,H7N7) and swine-origin H1N1(S- OIV)[J].
Virol J,2009,6:197.
[48] SHARMA M,ANDERSON S A,SCHOOP R,et al. Induc- tion of multiple pro-inflammatory cytokines by respiratory viruses and reversal by standardized Echinacea,a potent antiviral herbal extract[J]. Antiviral Res,2009,83(2): 165- 170.
[49] REINKE R A,LEE D J,MCDOUGALL B R,et al. L- chicoric acid inhibits human immunodeficiency virus type 1 integration in vivo and is a noncompetitive but reversible inhibitor of HIV- 1 integrase in vitro[J]. Virology, 2004,326(2):203- 219.
[50] CAPEK P,SUTOVSKá M,KOCMáLOVá M,et al. Chemical and pharmacological profiles of Echinacea complex[J]. Int J Biol Macromol,2015,79:388- 391.
[51] Zhang Shuo, Zhang Jianjun, Zhao Yimeng, et al. A literature review of the exotic medicinal herb Echinacea and a discussion of its “traditional Chinese medicine-ization” theory [J]. Chinese Journal of Traditional Chinese Medicine, 2020, 45(5): 978-983.
[52] VIMALANATHAN S,SCHOOP R,SUTER A,et al. Pre- vention of influenza virus induced bacterial superinfec- tion by standardized Echinacea purpurea,via regulation of surface receptor expression in human bronchial epi- thelial cells[J]. Virus Res,2017,233:51- 59.
[53] PARSONS J L,CAMERON S I,HARRIS C S,et al. Echi- nacea biotechnology:advances,commercialization and future considerations[J]. Pharm Biol,2018,56(1):485- 494.
[54] SHARIFI- RAD M,MNAYER D,MORAIS- BRAGA M F B,et al. Echinacea plants as antioxidant and antibacterial agents:from traditional medicine to biotechnological applications[J]. Phytother Res,2018,32(9):1653- 1663.
[55] TSAI Y L,CHIU C C,YI- FU CHEN J,et al. Cytotoxic effects of Echinacea purpurea flower extracts and cichoric acid on human colon cancer cells through induction of apoptosis[J]. J Ethnopharmacol,2012,143(3):914- 919.
[56] Han Lina, Kong Hao. A study on the correlation between the morphological traits, distribution height and quality of Echinacea purpurea [J]. Chinese Pharmacy, 2014, 25(7): 659-662.
[57] Sun Junying. A preliminary study on the morphology and dynamic accumulation of active ingredients in Echinacea purpurea [D]. Jinan: Shandong University of Traditional Chinese Medicine, 2011.
[58] Li Q, Chu C, Tan ZF, et al. Microscopic identification of Western herbal medicines I – microscopic identification of three types of Echinacea root [J]. Chinese Journal of Traditional Chinese Medicine, 2009, 34(21): 2718-2720.
[59] Wang D M, Li M, Li F. Microscopic identification of the medicinal plant Echinacea purpurea L. [J]. Shandong Agricultural Science, 2020, 52(6): 44-50.
[60] Luo X B, Chen B, Zhu X L, et al. HPLC determination of echinacoside in Echinacea purpurea and its compound preparations [J]. Traditional Chinese Medicine, 2002, 33 (10): 890-891.
[61] Kong Lingfeng. Research on the quality evaluation of domestically cultivated Echinacea herb [D]. Shenyang: Shenyang Pharmaceutical University, 2006.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese





