Study on Centella Asiatica Extract Active Ingredient Preparation Technology
Centella asiatica (L.) Urban. is the dried whole herb of the plant Centella asiatica (L.) Urban. in the family Apiaceae. Its active ingredients are mainly triterpene saponins and their derivatives [1], such as asiaticoside, madecassoside and madecassic acid, of which asiaticoside has the highest content. Asiaticoside has various pharmacological effects, such as promoting wound healing, treating scars, inhibiting breast hyperplasia, anti-tumor, anti-depression, and treating Alzheimer's disease [2]. The pharmacological activities of asiatic acid and madecassoside are similar to those of asiaticoside, while madecassoside also has the effect of protecting cardiovascular and cerebrovascular systems [3]. In order to better apply the active ingredients of Centella asiatica in clinical practice, domestic and foreign researchers have prepared various safe, stable and effective preparations for its various pharmacological activities. This paper summarizes the preparation research, summarizes the key technologies in the preparation process and the characteristics of the obtained preparations, and analyzes and summarizes the corresponding efficacy.

1 Injection
The titrated extract of Centella asiatica (TECA) injection can be used to treat systemic sclerosis. However, in a common aqueous solution, the solubility of TECA is low, and because the main component is a triterpene saponin, direct injection may cause hemolysis and a strong painful sensation. To solve the above problems, Chong-Kook et al. prepared a new TECA injection by adding Tween-20 and Tween-85 to the solution to improve the solubility of asiaticoside and reduce its toxic side effects [4]. Studies have shown that the higher the proportion of Tween-20 in the surfactant, the greater the solubility of TECA, up to 18 mg/ml. When the ratio of Tween-20 to Tween-85 is 6:4, the particle size of the dispersed phase in the prepared emulsion is the smallest. Compared with ordinary TECA propylene glycol injection, after the injection was administered to mice through intramuscular injection, the number of writhes of the mice was significantly reduced, indicating that the injection produced less pain, which is beneficial to increasing patient compliance and is more suitable for clinical application.
2 Oral preparations
2.1 Tablets
2.1.1 Sustained-release tablets
In order to solve the problems of the existing Centella asiatica tablets, such as the large dosage per serving and the large number of daily doses, Gan Kelin and others increased the dosage by 3 times on the basis of commercially available Centella asiatica tablets, and added hydroxypropyl methyl cellulose (HPMC) as a sustained-release material to to prepare the Centella asiatica total glycoside sustained-release tablets [5]. By using 20% K100M HPMC as the sustained-release carrier and adding 10% ethyl cellulose ethanol solution as the adhesion agent, sustained-release tablets were prepared, each weighing about 1.5 g and containing 18 mg of Centella asiatica total glycoside. The results of the in vitro release study showed that the ordinary Centella asiatica total saponins tablets had basically completely disintegrated and released all the drug after about 1 h, while the Centella asiatica total saponins sustained-release tablets could effectively release the drug for 12 h, achieving the purpose of slow and controlled release. Zheng Xiaochun et al. used K15M hydroxypropyl methylcellulose as a sustained-release carrier, microcrystalline cellulose as a release regulator, and a povidone ethanol solution as a binder to also produce a Centella asiatica total glycoside sustained-release tablet that can effectively release the drug for up to 12 hours [6].
2.1.2 Oral disintegrating tablets
In order to solve the problem of swallowing difficulties for some patients, Song Xuyin et al. prepared oral disintegrating tablets of asiaticoside [7]. By adding microcrystalline cellulose, cross-linked sodium carboxymethyl cellulose and mannitol, and using an orthogonal test design to optimize the prescription, the final oral disintegrating tablets can completely disintegrate within 1 min. At the same time, the tableting process is simple and easy to carry out, facilitating industrial production.

2.1.3 Gastric floating tablets
Studies have shown that asiaticoside has a good therapeutic effect on gastric ulcers [8]. Based on this, Shi et al. prepared asiaticoside gastric floating tablets that can remain in the stomach and prolong the drug delivery time [9]. By using sodium carboxymethyl starch as a bleaching aid, polyethylene glycol 20 000 as a pore-former, and a mixture design method to optimize the prescription, Centella asiatica total glucoside floating tablets that can effectively float for up to 12 h were finally obtained. Tableting has a significant impact on the appearance, floating performance and drug release performance of the tablet. Increasing the pressure can prolong the floating time, slow down the drug release rate and reduce the amount of drug released.
2.2 Suspension
Centella asiatica total glucoside has poor solubility in water, so it is often prepared as a suspension for oral administration. Literature shows that when used as a suspending agent with hydroxypropyl methylcellulose, it is administered to mice by gavage (30 mg/kg), and the degree of anxiety in mice is reduced in the elevated plus-maze experiment, the black box experiment, and the open field experiment. The anxiolytic effect of the drug becomes more pronounced with increasing dose [10]. At the same time, the preparation had a certain effect on the body weight of mice and the adrenaline content in the serum, but when the same dose of asiaticoside (10 mg/kg) or madecassoside (16 mg/kg) was used alone, there was no significant effect, indicating that the two may have a synergistic effect when used together, but it is also possible that the single use of a component is not sufficient in terms of dosage to produce an effect.

3 Topical preparations
3.1 Gels
Xia et al. used transdermal absorption rate and cumulative penetration as indicators to improve the transdermal absorption of asiaticoside by adding lauricine and geraniol to the prescription at different concentrations [11]. The results showed that the cumulative permeation and transdermal absorption rate could be increased by 3-12 times after adding a permeation enhancer, and the effect was best when 1% laurazone was used alone. Wang Shuping et al. also used borneol, menthol, etc. as permeation enhancers to improve the transdermal absorption of asiaticoside [12].
Surfactants can not only be used to increase the solubility of poorly soluble substances, but also promote the transdermal absorption of poorly soluble substances. Soon-Sun et al. investigated the solubilizing effect of a series of nonionic and anionic surfactants on TECA, and selected caprylic capric polyglycerol ester and sodium deoxycholate as solubilizers to prepare TECA gels [13]. A large amount of crystals appeared in the gel prepared with PEG polyoxyethylene glyceryl caprylate and caprate after 24 hours, while no obvious crystals appeared in the gel prepared with sodium deoxycholate after one month, indicating that the addition of sodium deoxycholate has a more stable effect on improving the solubility of asiaticoside. Animal experiments have shown that compared with the blank gel agent, the healing rate of mouse wounds is significantly faster and the efficacy is remarkable after using the TECA sodium deoxycholate gel agent.
3.2 Emulsions
Laugel et al. prepared a water-in-oil emulsion type composite emulsion with asiaticoside, madecassoside and asiatic acid (in a mass ratio of 4:3:3) as the main drug [14]. Tracer and rheological experiments showed that the prepared composite emulsion was stable, and that the presence of the drug had almost no effect on the stability of the emulsion compared to the blank emulsion. In vitro transdermal experiments showed that compared with ordinary oil-in-water emulsions, the complex emulsions had a retention rate in the epidermis and dermis that was three times and twice as high, respectively. When dimethicone is added to the outer oil phase, it can also improve the rate of drug absorption and produce a certain sustained-release effect [15]. At the same time, dimethicone with a lower molecular weight can improve the solubility of the drug in the stratum corneum, making triterpenoid drugs more likely to penetrate the stratum corneum and reach the epidermis, and increasing the amount of drug retained in the stratum corneum. Dimethicone with a higher molecular weight, however, will have the opposite effect.

3.3 Coating agent
The existing topical preparations of asiaticoside are mainly cream preparations, which have poor stability, a greasy feel, and are prone to staining clothing. In order to overcome these problems, Jiang Ping et al. used a chitosan aqueous solution as a base, supplemented with camphor, borneol, and menthol, to prepare an easy-to-use asiaticoside coating agent [16]. The content of asiaticoside and madecassoside in the film coating agent was 95% to 105% of the labeled amount, indicating that the prepared film coating agent had a stable drug content.
3.4 Membrane agent
Xin Tian et al. used deacetylated chitosan as the film-forming material to prepare a 6% asiaticoside film agent. The drug was evenly distributed in the film agent, and the drug release performance in vitro was good, with 90% of the drug released after 14 h [17]. The prepared film was used to treat uterine trauma in rabbits. Compared with the control group, the uterine trauma healed significantly after treatment with the Centella asiatica film, and there was no adhesion to the intestinal wall.
Panprung et al. used sodium alginate, a natural polysaccharide, as a raw material and prepared the centella asiatica film-forming agent by forming calcium alginate salt through complexation with Ca2+ as the matrix of the film-forming agent based on the traditional preparation method [18]. In this study, a high concentration of CaCl2 solution was used to carry out a secondary cross-linking reaction on the initially prepared film-forming agent to improve the stability of the film-forming agent. Mechanical property studies showed that the addition of asiaticoside could make the membrane withstand greater tensile stress, but it would make the membrane less flexible. In PBS containing 10% methanol, the membrane could effectively release the drug for up to 24 hours, and the cumulative release rate of asiaticoside could reach 92%. At the same time, toxicological studies showed that the membrane had no significant toxicity to fibroblasts in human dermis.
In contrast to the above preparation process, the process of directly preparing a film agent from sodium alginate, drying it, and then immersing it in a CaCl2 solution containing ethanol of different concentrations for cross-linking to obtain an asiaticoside film agent is simpler. Compared with a pure CaCl2 solution, the addition of the latent solvent ethanol can slow down the rate of cross-linking, resulting in a film with a more transparent appearance and a reduced thickness. Observed under a scanning electron microscope, the surface of the film agent with ethanol is more even, smoother, and has fewer folds. Studies have also found that when the concentration of ethanol is higher than 30%, it will inhibit cross-linking and is not conducive to the formation of a structurally stable film agent [19].
3.5 Babu agent
In order to increase the drug loading of topical preparations and improve the rate of drug release, Hu Xiao prepared a compound Centella asiatica saponin hydrogel cream (balm) with Centella asiatica total saponin and triamcinolone acetonide as the main drugs. A single-factor experiment was used to optimize the prescription, and the initial adhesion, holding power and skin followability of the gel cream were controlled at the optimal level [20]. In order to promote the absorption of asiaticoside, a permeation enhancer was added for in vitro experiments, and it was finally determined that 1% nitrocellulose and 1% propylene glycol were used as permeation enhancers for the compound asiaticoside gel cream. The prepared gel cream releases drugs quickly and stably, and the cumulative release amount is higher than that of the marketed control drug.
4 New preparation technologies and new dosage forms
The poor solubility and low bioavailability of the main active ingredient of Centella asiatica [21, 22] are not conducive to clinical application. To solve these problems, a series of new preparations and technologies have been widely used in the formulation research of Centella asiatica and its active ingredients.
4.1 Liposomes
Liposomes are supermicrosphere-shaped carriers composed of phospholipids and cholesterol with a bilayer structure similar to that of biological membranes. They can improve efficacy, reduce toxic side effects and prolong the duration of drug action [23]. Liu Minmin et al. used ethanol injection-high-pressure homogenization to prepare Centella asiatica glycoside-encapsulated flexible liposomes with an encapsulation rate of up to 91% [24]. The obtained nanoliposomes were used as a gelling agent based on methyl cellulose, which significantly increased the permeability and intradermal retention of asiaticoside in an in vitro transdermal experiment. Wang Huijuan et al. modified the hydroxyl asiaticoside liposome with polyethylene glycol to obtain a long-circulating liposome of asiaticoside, and optimized the prescription through response surface analysis. However, the encapsulation rate of the final liposomes was low, only 46% [25].
Donatella Paolino et al. used the film dispersion-extrusion method to prepare asiaticoside liposomes [26]. When lecithin and dipalmitoylphosphatidylcholine were used as the matrix, there was no significant effect on the encapsulation rate of asiaticoside liposomes. In vitro studies have shown that the content of cholesterol in the matrix has a significant positive effect on the penetration of asiaticoside into human epidermal fibroblasts, and that when the content is higher than 20%, it will produce a certain degree of cytotoxicity. In vivo studies have shown that compared with asiaticoside aqueous solution, the use of asiaticoside liposomes can effectively promote the transdermal absorption of asiaticoside and significantly increase collagen synthesis.
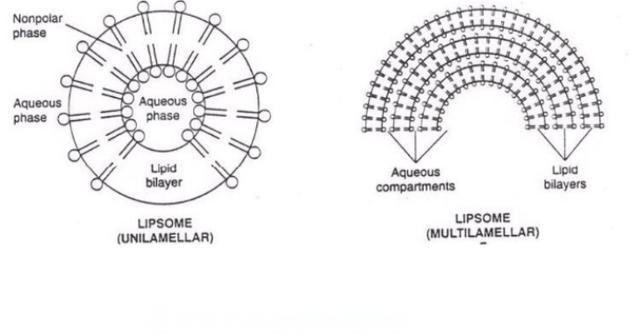
The author used the film dispersion method to prepare Centella asiatica glycoside liposomes and studied their modification (to be published). By modifying the liposomes with D-mannose and stearylamine, Centella asiatica glycoside cationic liposomes were prepared, with an encapsulation rate of up to 72.98%. The liposomes obtained showed a significant sustained-release effect in both in vitro and in vivo experiments; in particular, in vivo, the elimination half-life increased from 14.52 min to 159.58 min, and sustained drug release was achieved for more than 10 h. In a study of cholesterol levels, it was found that appropriately increasing the cholesterol content can significantly increase the encapsulation rate. It is speculated that cholesterol binds to asiaticoside [27], which improves the stability of asiaticoside in the phospholipid membrane. However, too high a cholesterol content can destroy the microscopic structure of the liposome [28], resulting in lumps in the final liposome and a decrease in the encapsulation rate.
4.2 Vesicles
Centella asiatica saponin is a triterpene saponin with a high molecular weight, high melting point, and poor water solubility and fat solubility. It is not easy to pass through the skin barrier for external use and has low absorption [29]. Zhang Yangyang et al. used nonionic surfactant and cholesterol as the encapsulating material to prepare vesicles by encapsulating centella asiatica saponin, and the entrapment rate of the obtained vesicles was 89.56% [30]. The results of an in vitro transdermal study showed that after 24 hours, the cumulative transdermal penetration of the Centella asiatica solution was only 3.73 μg/ml, while the cumulative transdermal penetration of the Centella asiatica vesicles was 25.15 μg/ml, with a cumulative transdermal penetration percentage of 88.95%.

4.3 Nanoemulsions
It has been reported that asiaticoside itself has a certain degree of targeting, and can be used to modify particulate drug delivery carriers. Sanghoon et al. used palmitoyl-lysine-threonine-serine-threonine-lysine (palmitoyl-KTTKS) as the main drug, asiaticoside as the targeting molecule, and Nile red as the marker to prepare palmitoyl-KTKS nanomilks [31]. Palmitoyl-KTTKS nanomilks were coated on isolated pig skin for in vitro transdermal experiments. After being left in the dark for 30 h, the skin was sliced and the distribution of the nanomilks in the skin was observed using a two-photon microscope and a laser scanning confocal microscope. The results showed that the transdermal depth of the asiaticoside-modified nanomilks was significantly higher than that of the unmodified nanomilks. This study confirmed that the asiaticoside-modified nanomilks can improve their affinity with stratum corneum cells and are more readily absorbed transdermally.
4.4 Encapsulation
Cyclodextrins and their derivatives have a cavity structure, and can encapsulate drug molecules through van der Waals forces, thereby improving the physicochemical properties of the drug. Zhang Bo et al. used hydroxypropyl-β-cyclodextrin (HP-β-CD) to prepare an inclusion complex with asiatic acid, increasing its solubility to 2.1 mg/ml [32]. The formation of the inclusion complex was confirmed by infrared spectroscopy, X-ray diffraction and differential scanning calorimetry. Scanning electron microscopy showed that the shape of the crystals obtained changed after the reaction.
Jate et al. found that when 2-hydroxypropyl-beta-cyclodextrin (2-HP-β-CD) was used to prepare an asiaticoside complex, the solubility of asiaticoside in distilled water increased from 0.7 mmol/L to 15.3 mmol/L at a 2-HP-β-CD concentration of 30 mmol/L, and the solubility in the mixed solvent (80% acetic acid solution: N-N-dimethylacetamide = 90:10) increased from 48.7 mmol/L to 60 mmol/L. The solubilization effect was linearly and positively correlated with the concentration of 2-HP-β-CD, and it was speculated that the host-guest molecular ratio in the inclusion complex was 1:1 [33]. The release of asiaticoside was improved by preparing the inclusion complex as a film with cellulose acetate as the membrane material. Soaking in PBS solution containing 10% methanol for 24 hours, the cumulative release of the complexes with a host-guest molecular ratio of 2:1, 1:1, and 1:2 reached 98%, 70%, and 38%, respectively, while the cumulative release of the asiaticoside film was almost 0.
4.5 Chitosan dressings
Chitosan is a polymer material with good biocompatibility and the effect of promoting wound healing, and is often used as an excipient for traumatic drugs. Kotchamon et al. improved the preparation method of chitosan dressings by dissolving chitosan in a lactic acid solution of aluminum stearate and supplementing with dehydrothymine treatment to improve the stability and microstructure of the dressing [34, 35]. The in vitro release of Centella asiatica extract made into a chitosan dressing formulation showed that the release of the Centella asiatica dressing conformed to a first-order kinetic equation, with a significant sustained-release effect and an effective drug release of up to 7 days. Cytotoxicity experiments showed that the prepared dressing was safe and non-toxic to human cells in vitro, and that the dressing could promote the division and reproduction of human fibroblasts and glial cells.

In summary, Centella asiatica has a wide range of pharmacological activities, and pharmaceutical research is of great significance for prolonging the duration of action of drugs, improving their efficacy, studying their mechanism of action, and improving their targeted distribution. Improving the absorption of the active ingredients in Centella asiatica through pharmaceutical means can enhance the therapeutic effect and make full use of Centella asiatica and its active ingredients.
References
[1] Liu Yu, Zhao Yuqing. Research on the chemical composition of Centella asiatica [J]. Chinese Modern Traditional Chinese Medicine, 2008, 10(3):7-9.
[2] Liu Zhifeng, Zhao Huinan, Nie Shaoliang, et al. Research progress on the pharmacological effects and mechanisms of asiaticoside [J]. Guangdong Medicine, 2009, 30(4):649-651.
[3] Liu Huaqing, Xu Qingyun, Wang Tianlin. Research progress on asiatic acid [J]. Chinese Wild Plant Resources, 2014, 33(4):30-33.
[4] Kim CK, Hwang YY, Chang JY, et al. Development of a novel dosage form for intramuscular injection of titrated extract of Centella asiatica in a mixed micellar system[J]. Int J Pharm, 2001, 220(1):141-147.
[5] Gan Kelin, He Wen. Preparation and in vitro release characteristics of Centella asiatica total glycoside sustained-release tablets [J]. Chinese Pharmacist, 2009, 12(6):714-716.
[6] Zheng Xiaochun, Wang Shenghao. Research on the prescription design of Centella asiatica total glycoside sustained-release tablets [J]. Chinese Journal of New Drugs, 2008, 17(11):958-961.
[7] Song Xuyang, Lu Jihong, Li Yingguang, et al. Development and quality control of oral disintegrating tablets of asiaticoside [J]. Chinese Herbal Medicine, 2011, 34(10):903-905.
[8] Shi Zhiqi, Du Jianping, Du Tieliang, et al. Study on the anti-gastric ulcer effect of Centella asiatica total saponins in rats induced by acetic acid [J]. Chinese Journal of Experimental Traditional Medicine, 2010, 16(12):122- 124.
[9] Shi Zhiqi, Wang Luolin, Du Jianping, et al. Optimization of the prescription process for Centella asiatica total glycoside floating stomach tablets [J]. Chinese Journal of Experimental Formulas, 2013, 19(12):15-16.
[10] Wanasuntronwong A, Tantisira MH, Tantisira B, et al. Anx- iolytic effects of standardized extract of Centella asiatica (ECa 233)after chronic immobilization stress in mice[J]. J Ethnopharmacol, 2012, 143(143):579-585.
[11] Xia Linhong, Qiu Qida. Effects of lauricine and menthol on the in vitro transdermal absorption of a compound asiaticoside gel [J]. Chinese Journal of Hospital Pharmacy, 2010, 30(18):1538-1541.
[12] Wang Shuping, Zhang Li, Zhang Li, et al. Effects of permeation enhancers on the in vitro transdermal penetration of asiaticoside [J]. Chinese Journal of Emergency Resuscitation and Disaster Medicine, 2014, 9(9): 816-819.
[13] Hong SS, Kim JH, Li H, et al. Advanced formulation and pharmacological activity of hydrogel of the titrated extract of C. Asiatica[J]. Arch Pharm Res, 2005, 28(4):502-508.
[14] Laugel C, Baillet A, Ferrier D, et al. Incorporation of triter- penic derivatives withinan O/W/O multiple emulsion: andre- lease studies[J]. IntJ Cosmetic Sci, 1998, 20(3):183-191.
[15] Laugel C, Rafidison P, Potard G, et al. Modulated release of triterpenic compounds from a O/W/O multiple emulsion formulated with dimethicones: infrared spectrophotometric and differential calorimetric approaches [J]. J Control Release, 2000, 63(1):7-17.
[16] Jiang Ping, Gao Hui, Wang Xiaomei, et al. Determination of asiaticoside and madecassoside in asiaticoside film coating agent by HPLC [J]. Pharmaceutical Service and Research, 2013, 13(4):282-284.
[17] Xin Tian, Zhang Jiali, Xia Wenshui. Preparation and efficacy study of asiaticoside-chitosan film agent [J]. Shaanxi Traditional Chinese Medicine, 2013, 34(7):901-902.
[18] Sikareepaisan P, Rulctanonchai U, Supaphol P. Preparation and characterization of asiaticoside-loaded alginate films and their potential for use as effectual wound dressings [J]. Carbohyd Polym, 2011, 83(4):1457-1469.
[19] Li J, He J, Huang Y, et al. Improving surface and mechanical properties of alginate films by using ethanol as a co-solvent during external gelation [J]. Carbohyd Polym, 2015, 123: 208-216.
[20] Hu X. Research on compound asiaticoside gel ointment [D]. Hubei University of Traditional Chinese Medicine, 2013.
[21] Zhang Y, Chen L, Zhang L, et al. Determination of the equilibrium solubility and oil-water partition coefficient of asiaticoside [J]. Journal of PLA Pharmacy, 2013, 29(3):189-191.
[22] Liu Shuyun, Fan Mingsong, Li Zhixiong, et al. Metabolism of madecassoside in rats [J]. Chinese Journal of Modern Traditional Chinese Medicine, 2012, 14(6): 9-15.
[23] Dua JS, Rana AC, Bhandari AK. Liposome: methods of preparation and applications [J]. Int J Pharm Stud Res, 2012, 3(2): 14-20.
[24] Liu Minmin, Li Li, Ma Xiaolai. Development and in vitro evaluation of Centella asiatica glycoside flexible nanoliposomal gel [J]. Chinese Journal of Pharmaceutical Industry, 2013, 44(11): 1120-1122.
[25] Wang HJ, Liu M, Du S. Optimization of madecassoside lipo- somes using response surface methodology and evaluation of its stability [J]. IntJ Pharm, 2014, 473(1):280-285.
[26] Paolino D, Cosco D, Cilurzo F, et al. Improved in vitro and in vivo collagen biosynthesis by asiaticoside-loaded ultradeformable vesicles [J]. J Control Release, 2012, 162(1):143-151.
[27] Wu Lijun. Natural Medicinal Chemistry [M]. Beijing: People's Medical Publishing House, 2012. 302-303.
[28] Wang Zhenzhi. The effect of cholesterol on the structure, properties and function of the bilayer membrane of liposomes [D]. Shanghai: East China University of Science and Technology, 2011, 48-52.
[29] Zhang Yangyang, Li Jian, Zhang Li. Evaluation of the effect of permeation enhancers on the in vitro transdermal drug release characteristics of asiaticoside using the residence time method [J]. Chinese Journal of Emergency Care and Disaster Medicine, 2013, 8(7):623-625.
[30] Zhang Yangyang, Zhao Shangqing, Chen Li, et al. Determination of the encapsulation rate of centella asiatica vesicles by equilibrium dialysis [J]. Journal of the Armed Police Logistics College (Medical Edition), 2015, 24(12): 951-954.
[31] Choi S, Kim J , Lee YJ, et al. Evaluation of transdermal de- livery of nanoemulsions in ex vivo porcine skin using two- photon microscopy and confocal laser-scanning microscopy [J]. J Biomed Opt, 2014, 19(10):106006-106006.
[32] Zhang B, Zhao YL. Study on the solubilization of asiaticoside by hydroxypropyl-β-cyclodextrin [J]. Journal of Tianjin Medical University, 2014, 20(6):486-489.
[33] Panichpakdee J, Supaphol P. Use of 2-hydroxypropyl-β-cyclodextrin as adjuvant for enhancing encapsulation and release characteristics of asiaticoside within and from cellulose acetate films [J]. Carbohyd Polym, 2011, 85(1):251-260.
[34] Yodlchum K, Phaechamud T. Hydrophobic chitosan sponges modified by aluminum monostearate and dehydrothermal treatment as sustained drug delivery system [J]. Mater Sci Eng C, 2014, 42:715-725.
[35] Phaechamud T, Yodkhum K, Charoenteeraboon J, et al. Chitosan- aluminum monostearate composite sponge dressing containing asiaticoside for wound healing and angiogenesis promotion in chronic wound [J]. Mater Sci Eng C, 2015, 50: 210-255.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






