Study on Black Rice Anthocyanin
Black rice anthocyanin (anthocyanin) is a flavonoid polyphenol compound that is a glycoside formed by the combination of anthocyanidin and various sugars via glycosidic bonds. It is found in the cell sap of the fruit, stems and leaf organs of black rice [1-2]. Guo Honghui et al. [3] found in their research that anthocyanin pigments accumulate in the seed coat during the ripening process of black rice, giving the brown rice a brownish red, purple red, purple black or even black color. Zeng Gui et al. [4] found that in addition to giving plants rich colors, anthocyanins also have physiological functions such as anti-oxidation, anti-inflammation, lowering blood lipids, and inhibiting tumor growth. At the same time, anthocyanins, as a relatively safe natural pigment, also show broad application prospects in the food industry.
Zhong Yan et al. [5] believe that the physiological health benefits of black rice are mainly related to the black rice anthocyanin pigment that is abundant in black rice. As the safety of synthetic pigments commonly used in the food processing industry is of increasing concern, the development and research of natural pigments has become a hotspot in the field of food research. With the rise of black foods and the industrial production and application of black rice anthocyanin pigments, the demand for black rice anthocyanin in the food industry is increasing. Therefore, it is necessary to carry out research on the composition analysis of anthocyanin types in different black rice, pharmacological research on anthocyanins, and the industrial production technology and stability of black rice anthocyanin. This paper provides a review of research on black rice anthocyanins in China in recent years.
1 Research on the composition of black rice anthocyanins
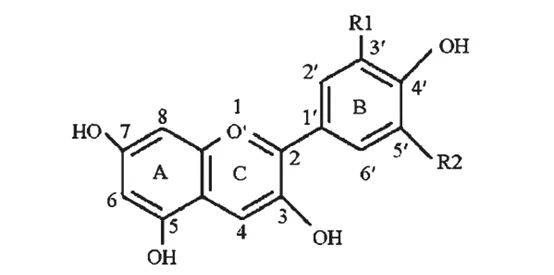
Black rice anthocyanin is a glycoside compound formed by anthocyanins binding to various monosaccharides in a natural state, and generally several anthocyanins coexist [6]. Zhong Liyu et al. [7] used black whole-stem 91-53 provided by the Shanghai Academy of Agricultural Sciences as the material, and used paper chromatography, ultraviolet-visible scanning and gas chromatography combined methods to identify the molecular structure of black rice pigments. The results showed that black rice pigment is composed of five compounds, and two of them have been identified as cyanidin-3-rutinoside and peonidin-3-arabinoside. Su Jin et al. [8] used brown rice from two black rice varieties, Yanwhei and Yanwuzi, and identified anthocyanin-3-glucose and anthocyanin-3-rhamnosyl glucose as the components of the pigment through component separation and identification. Xia Xiaodong et al. [9] used high performance liquid chromatography to determine the composition and content of anthocyanin in black rice bran extract. The results showed that the contents of cyanidin-3-glucoside and peonidin-3-glucoside in black rice extract were 25.7% and 1.7%, respectively.
Zhang et al. [10] performed structural analysis on black rice extract, which showed that black rice extract contains four anthocyanin compounds, namely, malvin, geranin-3.5-diglucoside, centaureidin-3-glucoside and centaureidin-3, 5-diglucoside. Based on the analysis of the physical and chemical properties of black rice pigments, it is inferred that black rice pigments (glycosides) are dominated by anthocyanins and cyanidin.
Zhang Fudi et al. [11] showed that the basic structure of black rice anthocyanin glycosides is similar to that of cyanidin-3-glucoside and cyanidin-3-rutinoside. Wang Qing et al. [12] used isocratic elution reversed-phase high performance liquid chromatography to analyze the anthocyanins in black rice bran and the metabolic conversion of these pigments in the human body. The results showed that the total anthocyanin content in black rice bran was about 2.31%, of which cyanidin-3-glucoside accounted for 1.87% and peonidin-3-glucoside accounted for 0.44%. Kong Lingyao et al. [13] used liquid chromatography-mass spectrometry (LC-MS) and capillary electrophoresis-electrochemical detection (CE-ED) to qualitatively analyze black rice pigments, and the results showed that the components of black rice pigments were respectively cyanidin-3-glucoside and peonidin-3-glucoside. Park et al. [14] used high-performance liquid chromatography and ultraviolet-visible spectrophotometry to qualitatively and quantitatively analyze anthocyanin extracts.

The results showed that black rice anthocyanins include cyanidin-3-glucoside, anthocyanin-3-glucoside, malvin-3-glucoside, pelargonidin-3-glucoside and delphinidin-3-glucoside. Of these, the content of cornflower blue 3-glucoside is about 95%, and the content of cyanidin 3-glucoside is about 5%. Mikihle-mori et al. [15] used high performance liquid chromatography-photodiode array detection (HPLC-PDA) and electrospray mass spectrometry to investigate the composition and thermal stability of black rice pigments. The results showed that the main components of black rice anthocyanin pigments were cyanidin-3-glucoside (572.47 μg/g, 91.13%) and cyanidin-3-glucoside (29.78 μg/g, accounting for 4.74%). Konishi [16] used a 3% trifluoroacetic acid solution to extract anthocyanins from purple black rice. Capillary electrophoresis showed that there was an anthocyanin in the purple black rice extract, which was determined to be cyanidin-3-glucoside after column chromatography purification.
In summary, the components of the pigment extracts from black rice, black rice husk or black rice straw are: cyanidin-3-glucoside, cyanidin-3,5-diglucoside, cyanidin-3-rutinoside, peonidin-3-glucoside, paeoniflorin-3-arabinoside, pelargonidin-3,5-diglucoside, malvin, malvin-3-galactoside, etc. There are 8 types in total. Due to the differences in black rice varieties, material processing methods, and extraction purposes, the anthocyanin content of black rice extracts in the market also varies. At present, there are very few research reports on the analysis and comparison of Anthocyanin components in different black rice varieties.
2 Pharmacological research on black rice Anthocyanin
The main component of black rice extract is the flavonoid Anthocyanin compound, which has a variety of physiological functions [6]. Hu Qiulin [17] used purified black rice pigment as the material and conducted an animal nutrition experiment using mice. The results showed that black rice extract can improve its anti-fatigue and anti-hypoxia abilities. Xia Xiaodong et al. [18] studied the effect of black rice anthocyanin extract on advanced atherosclerotic plaques in ApoE gene-deficient mice. The results showed that black rice anthocyanin extract can significantly reduce the levels of total cholesterol, total triglycerides and low-density lipoprotein cholesterol in mouse serum, while also reducing the plaque area in the unnamed artery and the content of matrix metalloproteinase in the plaque. This indicates that black rice anthocyanin extract can inhibit the further development of advanced atherosclerotic plaques in mice.
Yang Jingya et al. [19] believe that anthocyanins are flavonoids with very good antioxidant effects, which can effectively inhibit the infiltration and metastasis of cancer cells. Qin Yu et al. [20] observed the clinical efficacy of black rice anthocyanin extract capsules in the treatment of hyperlipidemia. The results showed that black rice anthocyanin extract capsules have a significant adjuvant lipid-lowering effect. Hu Yan et al. [21] studied the effect of black rice anthocyanin extract on obesity induced by a high-fat diet in rats. The results showed that black rice anthocyanin extract can improve obesity-related indicators in rats induced by a high-fat diet.
Hu et al. [22] used an in vitro model to demonstrate that anthocyanin extracted from black rice has the same antioxidant activity and free radical scavenging capacity as a mixture containing known proportions of cornflower-3-glucoside and cyanidin-3-glucoside; it can also reduce cytotoxicity by inhibiting the expression of nitric oxide synthase in mouse macrophages. This study shows that anthocyanins, which contain antioxidant and anti-inflammatory properties, have great potential for use in the formulation of health foods or functional foods.
Itani et al. [23] compared the antioxidant activity and the different distribution of active substances in six rice varieties (two red rice varieties, two purple-black rice varieties and two white rice varieties). Compared to white hulled rice, the ethanol extracts of red and purple-black hulled rice had a higher capacity to scavenge superoxide anions and free radicals. Most of these active substances are found in the pericarp and seed coat, i.e. the bran. Colored rice is much richer in polyphenols than white rice, and the content is correlated with its antioxidant effect. In red and purple rice, the main active substances are tannins and anthocyanins, respectively. Zhang et al. [24] analyzed the total antioxidant capacity and the ability to scavenge active oxygen radicals of black rice and its correlation with the total flavonoid and anthocyanin content. There was a highly significant (P<0.01) positive correlation between the total antioxidant capacity and free radical scavenging capacity of black rice and the total flavonoid and anthocyanin content, indicating that the antioxidant effect of black rice is closely related to the flavonoid and anthocyanin substances it contains.
In addition, black rice pigments have a chelating effect on iron, ketone, zinc, etc. Regular consumption of black rice or black rice products can supplement iron and prevent iron deficiency anemia. At present, most pharmacological studies are in the animal testing stage. Due to the complex composition of black rice anthocyanin extracts, it is not yet clear whether the pharmacological effects are due to a single ingredient or a combination of several ingredients.
3 Extraction and purification of black rice anthocyanin
3.1 Extraction of black rice anthocyanin
The extraction of black rice pigments is affected by many factors such as extraction time, temperature, material-to-liquid ratio, solvent, pH, etc. Wang Yinding et al. [25] conducted an orthogonal experiment on the factors affecting the extraction of black rice pigments and obtained the optimal combination of conditions as extraction temperature 40 °C, extraction time 1 h, material ratio 1:50, and extraction agent 50% ethanol solution. Liu Jinglan et al. [26] studied the extraction method of black rice pigment and the effects of factors such as acidity, temperature, and light on its stability. The results showed that at around 65 °C, the higher the acidity and the longer the soaking time, the greater the extraction yield. The pigment was extracted twice with 0.05 mol/L hydrochloric acid solution to obtain a pigment gel with an extraction rate of about 5.0%.
Liu Feng et al. [27] added black rice to an extraction tank and allowed it to stand and soak in water at 20–40 °C; an ethanol solution was added separately and heated to 40–50 °C; the mixture was extracted by circulation, concentrated by vacuum distillation, and spray-dried to obtain a powdered anthocyanin product. Zhao Quan et al. [28] used black rice as the raw material and carried out single factor experiments and orthogonal experiments to determine the optimal extraction conditions. The results showed that the best extraction conditions were a 75% ethanol solution, a liquid-to-material ratio of 1:8, a temperature of 30 °C, and a time of 30 min. Under these conditions, the black rice anthocyanin extraction rate could reach 94.40%. Zhang Mingwei et al. [29] used the optimal process conditions of 60% ethanol solution, a material-liquid ratio of 1:4, a temperature of 60 °C, and a time of 4 h. Under these conditions, the first extraction yield was 71.4%, the second extraction yield was 13.63%, and the total of the two extractions was over 85%.
Huang Lisha et al. [30] used ethanol-water as a solvent to extract black rice pigments from black rice straw. The results showed that at pH 2, 70 °C, 60 min, and 60% ethanol solution, the extraction was the most effective, with an extraction rate of 31.59%. Zeng et al. [4] used black rice as the raw material and studied the factors that affect the extraction rate of black rice pigments (type, concentration, temperature, pH value, time, etc. of the extraction agent). The results showed that the highest extraction rate was obtained with a 70% ethanol solution (pH 2) at 70 °C for 90 min each time. Zhang Fudi et al. [11] studied the process steps for preparing black rice pigment from dietary black rice. The optimal extraction temperature obtained using the orthogonal test method was 80 °C, the time was 30 min, the liquid-to-material ratio was 1:10, and the extraction agent was a 50% ethanol solution. Zhong Yan et al. [5] studied the extraction conditions of melanin, and the results showed that the optimal extraction conditions were 50% ethanol solution, material to liquid ratio 1:10, pH 1.0, and extraction in an 80°C water bath for 30 minutes.
At present, the main materials for the extraction of anthocyanin substances from black rice are black rice, black rice husk and black rice straw. The extraction solvents generally chosen are ethanol (mostly 50%-80% ethanol solution) and a small amount of inorganic acid (hydrochloric acid, sulfuric acid, etc.) or organic acid (citric acid, acetic acid, etc.). Anthocyanin is found in the vacuoles of plant cells, wrapped by cell walls and cell membranes. To increase the pigment yield, heating, enzymes (such as amylase, pectinase, cellulase and protease, etc.), ultrasound, mechanical crushing, microwaves, freezing and pulsed electric fields are used to break down cell walls and cell membranes, increase the permeability of tissue cells, shorten extraction times, increase pigment yields and improve product quality. These auxiliary methods are often used in combination during the application process, which can increase the yield of pigments, but the conditions for use of auxiliary technologies, energy consumption and other issues still require further research.

3.2 Research on the separation and purification of anthocyanin from black rice
The main technologies that can be used for the separation and purification of anthocyanin from black rice include macroporous resin separation technology, gel chromatography, high-speed countercurrent chromatography, and membrane technology. Zhang Mingwei et al. [29] used total antioxidant capacity as an activity tracking indicator, and selected petroleum ether or hexane for degreasing when removing impurities from the antioxidant extract of black rice bran. Through a comparison of static and dynamic adsorption properties, among the eight types of macroporous adsorption resins, the one with the best adsorption capacity for the antioxidant active substances in black rice bran was selected as NKA-II, and the best desorbent was a 70% ethanol solution. After adsorption and separation by NKA-II, the total antioxidant capacity of the black rice skin antioxidant extract increased by 4.00 times, and the total anthocyanin content increased by 4.01 times. Zhang Qing [31] found that after purification with macroporous adsorption resin, the anthocyanin content of black rice pigment can reach 23.7%, with a color value of 83, and the purity is much higher than the current national standard. Hou Fangli et al. [32] compared the adsorption and purification effects of five types of macroporous adsorption resin on black rice skin anthocyanin. The results showed that AB-8 macroporous resin has better adsorption and desorption capacity for black rice skin anthocyanin and is the best type of resin for the adsorption and purification of black rice skin anthocyanin. The optimal process parameters are: pH 2 for the upper column liquid, a sample mass concentration of 1.0 mg/mL, an adsorption flow rate of 1.0 mL/min, 70 % ethanol as the desorbent, and an elution rate of 1.0 mL/min.
At present, the use of macroporous resin has become the mainstream of the separation and purification of black rice anthocyanin pigments. Because the black rice pigment solution extracted by the solvent method still contains a lot of impurities such as sugars and organic acids, the product quality is poor, the stability is low, and it is difficult to apply. In order to obtain a product with high purity and stable quality, the extraction technology and purification methods need to be further improved.
4 Stability study of black rice anthocyanin
The stability of black rice anthocyanin involves the structure, concentration, and quality of the anthocyanin itself, as well as external factors such as light intensity, temperature, pH, sulfur dioxide, co-colorants, enzymes, ascorbic acid, sugars and their degradation products, metal ions, and the combined effects of metal ions on molecular polymerization, isomerization, and degradation. Wang Feng et al. [2] believe that the aglycone of anthocyanin is a polyhydroxy and methoxy derivative of the 2-phenylbenzopyran cationic structure or xanthone salt. The lack of electrons makes it highly reactive, and the multiple hydroxyl groups attached to the parent nucleus make it unstable. The specific manifestation of the change in the stability of anthocyanins is a change in color. The fading and imbalance of black rice anthocyanins seriously affects its application.
Li Lirong et al. [33] used colorimetry to study the effects of external factors and five sterilization processes on the stability of anthocyanin in the seed coats of black rice, black soybeans and black corn. Under conditions of light avoidance, natural light and fluorescent light, the anthocyanin in black soybeans was the most stable, followed by the anthocyanin in black rice, and the anthocyanin in black corn was the least stable. Under the same temperature conditions, the anthocyanin in black rice and black soybeans was more stable, while the anthocyanin in black corn was less stable. Kong Lingyao et al. [13] studied the modification of pigment structure, and found that supplementing with accessory pigments, acylation, complexation with certain metal ions, and glycosylation with anthocyanins can improve the stability of black rice anthocyanins. The color protection in food is mainly achieved by the co-color protection of anthocyanins. These studies are of great significance for improving the stability of pigments and their application in food processing.
To improve the stability of anthocyanin, the main methods are to keep anthocyanin pigments in an acidic environment, avoid light as much as possible, store at a lower temperature, and add suitable stabilizers. In terms of pigment structure modification, the main research contents include supplementing with auxiliary colorants, acylation, metal ion complexation, and glycosylation with anthocyanidin glycosides, etc., to improve the stability of black rice pigments. However, the stability of black rice anthocyanin in food and pharmaceutical industry applications still requires further research.
5 Outlook
In short, black rice anthocyanin pigments, as a natural food coloring, are safe, non-toxic, odorless, brightly colored, abundant in resources, and have certain nutritional and pharmacological effects. They have great application potential in food, medicine, cosmetics, and other fields. However, the industrial production of black rice anthocyanin pigments is still in its infancy.

At present, there are few research reports on the analysis and comparison of Anthocyanin components in different black rice varieties; most pharmacological studies are in the animal testing stage, and it is not yet clear whether the pharmacological effect is caused by a single component or a combination of several components; in order to obtain a product with high purity and stable quality, the extraction technology and purification method for black rice Anthocyanin need to be further improved.
It is still unclear whether the pharmacological effect of anthocyanin is due to a single component or a combination of several components. In order to obtain a product with high purity and stable quality, the extraction technology and purification method of black rice anthocyanin need to be further improved. It is still the focus of future research to clarify the type, distribution and content of anthocyanins in different black rice varieties, screen black rice varieties with high anthocyanin content and good stability, clarify the pharmacological effects of black rice anthocyanin, and develop new extraction, separation and purification technologies to better remove impurities and improve pigment quality, and solve the stability of black rice pigment application.
References:
[1] Wu Sanqiao, Shi Suixiao, Ding Rui, et al. Research on the determination method of anthocyanin pigments in black rice [J]. Amino Acids and Biological Resources, 2002, 24(3): 66-68.
[2] Wang Feng, Deng Jiehong, Tan Xinghe, et al. Research progress on anthocyanin and its co-color effect [J]. Food Science, 2008, 29(2): 472-476.
[3] Guo Honghui, Ling Wenhua. Research progress on black rice anthocyanin [J]. Food Research and Development, 2008, 29( 3) :133-135.
[ 4] Zeng Kui, Huang Bin, Wang Jie, et al. Extraction and purification of black pigments from black rice [ J] . Food Science, 2006, 27( 12) :304-307.
[ 5] Zhong Yan, Li Zehong, Shao Mingfu. Extraction method and stability study of black rice pigment [J]. Northern Gardening, 2008, (10): 71-73.
[6] Cao Xiaoyong, Li Xinsheng. Research status and prospects of anthocyanin pigments in black rice [J]. Amino Acids and Biological Resources, 2002, 24(1): 3-6.
[7] Zhong Liyu, Hu Qiulin. Molecular structure analysis of black rice pigment [J]. China Cereals and Oils Journal, 1996, 11(6): 26-35.
[8] Su Jinwei. Extraction and component analysis of black rice pigment [J]. Journal of Fujian Agriculture and Forestry University, 1999, 28(2): 22-26.
[9] Xia Xiaodong, Ling Wenhua, Zheng Lin, et al. Determination of anthocyanin components and content in black rice bran extract by HPLC [J]. Food Science, 2006, 27(2): 206-208.
[10] Zhang Ming-wei, Guo Bao-jiang, Zhang Rui-fen et al. Separation, purification and identification of antioxidant compositions in black rice [J]. Agric Sci China, 2006, 5(6): 153-160.
[11] Zhang Fudi, Su Jinyi, Cai Biqiong. Extraction process and characterization of black rice pigments [J]. Journal of Fujian Agriculture and Forestry University: Natural Science Edition, 2006, 35(1): 93-97.
[12] Wang Qing, Guo Honghui, Zhang Mingwei, et al. Analysis of anthocyanins in black rice bran and their metabolites in vivo by high performance liquid chromatography [J]. Food Science, 2006, 27(5): 212-215.
[13] Kong Lingyao, Wang Yun, Cao Yuhua, et al. Composition and structural analysis of black rice pigments [J]. Journal of Food and Biotechnology, 2008, 27(2): 25-29.
[14] Park Y S, Kim S J, Chang H I. Isolation of anthocyanin from black rice (Heugjinjubyeo) and screening of its antioxidant activities [J]. Korean J Microbiol Biotechnol, 2008, 36(1): 55-60.
[15] Mikihlemori, Eunmikoh, Alysone M. Influence of cooking on antho- cyanins in black rice (Oryza sativa L. japonica var. SBR) [J]. J Agric Food Chem, 2009, 57(5): 1908-1914.
[16] Konishi T. Antioxidant activity of anthocyanin extract from purple black rice [J]. J Med Food, 2001, 4(4):211-218.
[17] Hu Qiulin. Research report on pharmacological animal experiments of black rice pigments [J]. Journal of Wuhan Institute of Food Industry, 1997, (3): 10-12.
[18] Xia Xiaodong, Ling Wenhua, Xia Min, et al. Effects of black rice anthocyanin extract on advanced atherosclerotic plaques in ApoE gene-deficient mice [J]. Food Science, 2006, 27(3): 213-215.
[19] Yang Jingya, Wu Hongzhong, Hu Yi, et al. Natural antioxidants—a new way to fight cancer [J]. Chinese Journal of Clinical Pharmacology and Therapeutics, 2007, 12(4): 366–370.
[20] Qin Y, Ling W. The lipid-lowering effect of black rice anthocyanin extract capsules in patients with hyperlipidemia [J]. Food Science, 2008, 29(10): 540-542.
[21] Hu Yan, Guo Honghui, Wang Qing, et al. Effects of black rice anthocyanin extract on obesity induced by high-fat diet in rats [J]. Food Science, 2008, 29(2): 376-379.
[22] Hu C, Zawistowski J, Ling W H, eta1. Black rice (Oryza sativa L. indica) pigmented fraction suppresses both reactive oxygen species and nitric oxide in chemical and biological model systems [J]. J Agric Food Chem, 2003, 51(18):5271-5277.
[23] Itani T, Tatemoto H, Okamoto M, et al. A comparative study on antioxidant activity and polyphenol content of colored kernel rice Japanese [J]. Japan Society of Food Science and Technology, 2002, 49(8): 540-543.
[24] Zhang Ming-wei, Guo Bao-jiang, Chi Jian-wei, et al. Antioxidations and their correlations with total flavones and anthocyanin contents in different black rice varieties [J]. Agric Sci China, 2005, 38(7): 1324-1331.
[25] Wang Yinding, Wang Zhaohui, Yan Shuling. Study on the extraction conditions and properties of black rice red pigment [J]. Journal of Hebei University: Natural Science Edition, 1995, 15(4): 101-104.
[26] Liu Jinglan, Chen Lianwen. Preliminary study on the extraction and stability of black rice pigment [J]. Journal of Hebei Normal University: Natural Science Edition, 1995, 19(2): 71-74.
[27] Liu Feng, Li Chong, Zhang Jilu. A method for extracting the natural pigment anthocyanin from black rice: China, 200710056592[P]. 2008-11-12.
[28] Zhao Quan, Wang Jun. Study on the extraction process of anthocyanin from black rice [J]. Anhui Agricultural Science, 2009, 37(3): 920-921.
[29] Zhang Mingwei, Guo Baojiang, Chi Jianwei, et al. Extraction and separation process of antioxidant active substances from black rice husk [J]. Transactions of the Chinese Society of Agricultural Engineering, 2005, 21(6): 135-139.
[30] Huang Lisha, She Xiaoman, Peng Liping, et al. Extraction of black rice pigment from black rice straw [J]. Journal of Shaoguan University: Natural Science Edition, 2003, 24(6): 59-61.
[31] Zhang Q. Study on the extraction and purification process of black rice pigment [J]. Journal of Qingdao University, 2000, 15(2): 24-26.
[32] Hou Fangli, Zhang Mingwei, Su Dongxiao, et al. Study on the adsorption and purification of black rice skin anthocyanin by macroporous resin [J]. Journal of South China Normal University: Natural Science Edition, 2009, 1:100-104.
[33] Li Lirong, Zhang Mingwei, Liu Linwei, et al. Stability comparison of anthocyanin in the seed coats of three black crops [J]. Transactions of the Chinese Society of Agricultural Engineering, 2007(5): 391-395.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






