Oat Beta Glucan, What Is It?
Glucan is a polymer of dextrorotatory pyranose with the molecular formula (C6H10O5)n. The backbone is formed by glucosidic bonds between the carbon 1, 2, 3, 4 and 6 of adjacent glucose residues, with two structural forms: α and β positions [1]. β-Glucan is named after its β-1,3 glycosidic bond [2]. In addition to the basic structural characteristics of a main chain and branches, β-glucan also has a higher-level structure with spiral characteristics. High molecular weight β-1,3-glucan mainly exists in the form of two advanced structures: 1-helix and 3-helix. It also exists in the form of random loops composed of low molecular weight or charged molecules [3].
β-glucan is widely found in plants and microorganisms, and is an important component of cell walls. It exists in various forms due to differences in molecular weight and branching degree [4], as shown in Table 1.
Langerhans cells, which are distributed in the prickly layer of the epidermis and between the cells of the basal layer, can capture and process antigens that have invaded the skin and transmit them to T cells, which can cause specific T cells to proliferate and activate. β-1,3-D-glucan can specifically bind to Langerhans cells, causing a series of immune responses, which in turn produce cytokines such as granulocyte-macrophage colony-stimulating factor (Colony-stimulating factor, GM-CSF), epidermal growth factor (EGF), fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF) and other cytokines [9].
GM-CSF can stimulate cell differentiation and enhance the function of mature cells; an increase in EGF can not only activate the activation and expression of some important functional genes in cells, increase the production of collagen and elastin, thereby improving the problem of wrinkles caused by skin aging, and also stimulate the proliferation and migration of various cells to accelerate the turnover of the epidermis; FGF can promote the proliferation of smooth muscle cells and the formation of new blood vessels to repair damaged skin; VEGF is involved in maintaining normal blood vessel structure and regulating physiological and pathological angiogenesis [10].
In addition, the administration of granular dextran can enhance the hematopoietic activity of blood cells, including the production of granulocytes, mononuclear leukocytes and red blood cells, thereby leading to recovery from near-lethal doses of radiation [11], which is a promising and noteworthy function. The disadvantage of beta-glucan is that it may cause inflammation of the respiratory tract, trigger allergies and be associated with hay fever syndrome [4].
1 Physical and chemical properties of oat beta-glucan
Oat β-glucan is a high-molecular, unbranched, linear mucopolysaccharide formed by β-(1,3) and β-(1,4) glycosidic bonds linking β-D-glucose units, with about 70% β-(1,4) bonds and 30% β-(1,3) bonds [12].
Although the mechanism of β-glucan is not yet fully understood, it is generally agreed that viscosity and solubility play a decisive role in its skin absorption effect, which in turn affects the performance of various physiological functions. In addition to the molecular structure and concentration, the viscosity of oat β-glucan is largely determined by the molecular weight and molecular shape [13]. The viscosity of an oat β-glucan solution decreases gradually with increasing shear rate; it is proportional to the molecular weight and inversely proportional to the solution temperature. Compared with a neutral solution, a weakly acidic or alkaline environment can cause the viscosity of a β-glucan solution to decrease. As its concentration increases and its molecular weight increases, the viscous behavior of the fluid decreases and the elastic behavior increases. As the fluid temperature increases, the viscosity and elasticity of oat β-glucan fluids gradually weaken [14].
Studies have shown that the water solubility (which is the majority) and non-water solubility of β-glucan are mainly affected by the content and degree of polymerization of the β-(1,3) glycosidic bonds in its structure. The ratio of the content of β-(1,3) glycosidic bonds to β-(1,4) glycosidic bonds in water-soluble β-glucans is 1:2.5 to 1:2.6, while the corresponding ratio in non-water-soluble β-glucans is 1:4.2 [15].
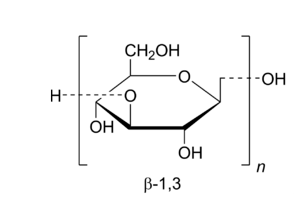
As shown in Figure 1, both oat beta-glucan and yeast beta-glucan are glucose polysaccharides with β-1,3-glucosidic bonds as the main chain, but the side chain of oat beta-glucan is β-1,4-glucosidic bond, while the side chain of yeast beta-glucan is β-1,6-glucosidic bond. Peterson et al. found that the ratio of β-(1,3) to β-(1,4) glycosidic bonds in oat β-glucan is (1:2.1–1:2.4), while the ratios in barley, rye, and wheat: 1: 2.8–1: 3.3, 1: 3.0–1: 3.2, and wheat: 1: 3.0–1: 3.8, respectively [16]. It is therefore clear that oat β-glucan has a stronger water solubility than yeast β-glucan, which has the largest market share, and other grains.
Currently, the β-glucan powder used in cosmetics is mostly insoluble yeast β-glucan solid particles (D=0.2 μm), usually with sorbitol as an effective suspending agent, and is generally used for wound healing. Carboxymethylation of yeast β-glucan can improve the water solubility of the product, making it suitable for use in modern functional cosmetic formulations. However, it also affects the 3-D structure and biological function of the molecule: when the degree of substitution of carboxymethylation exceeds 75%, the biological function begins to be lost; complete substitution of the β-glucan molecule leads to a complete loss of its biological efficacy [9]. From this perspective, the development of oat β-glucan is of great significance for the application of β-glucan in cosmetics.
Oat β-glucan solutions in the concentration range below 1% shows good homogeneity and viscous fluid properties, and is an ideal Newtonian fluid. When the concentration reaches 2%, β-glucan shows some heterogeneity and viscoelasticity [17]. When the concentration reaches 2 g/L or more, it has the characteristics of a pseudoplastic fluid, that is, the apparent viscosity value decreases with an increase in shear rate, laying the foundation for its use as a thickener and stabilizer [12].
Secondly, oat β-glucan is relatively stable to heat, acids and alkalis, and has been used in the food industry as a good emulsifier, thickener and stabilizer. It has even been added to meat products to improve the texture due to its good water and oil retention properties[18]. Furthermore, oat β-glucan has a strong ability to adsorb small molecules, which can compete with proteins. It combines with polyphenols through hydrogen bonds and hydrophobic interactions, etc., to form a polysaccharide-polyphenol complex that can provide the body with more lasting antioxidant capacity [19]. Mark Redmond and Ravi Pillai, Joachim Roding, etc., used human skin model dye fluorescence tracking experiments to find that oat β-glucan molecules can pass through the intercellular space and have obvious penetration of the epidermis. These characteristics also indicate that the application of oat β-glucan has broad prospects.

2 Influencing factors on the extraction effect and characteristics of oat β-glucan
Different oat qualities, the environment in which the oats grow, the processing and extraction processes, these four factors have a different impact on the content and physicochemical properties of oat β-glucan.
2.1 Differences between oat varieties
Studies have shown that the β-glucan content of different species of oats varies greatly, with the content of naked oats (A. nuda, large-grained naked oats, also known as oat groats) higher than that of hulled oats (Sativa, commonly cultivated oats, commonly known as oats). The ratio of soluble β-glucan to total content also shows significant species differences, with the skin oat species having higher values than the naked oat species at all times [20]. The β-glucan content of different oat varieties varies from 3.14% to 7.43%, with a maximum difference of 4.29% [21]. Zhang Haifang et al. used the Congo red spectrophotometry method to determine the β-glucan content of 16 different varieties of oat grains grown in Wuchuan and Zhaoshan, Inner Mongolia. Among them, there were 7 varieties with β-glucan content above 6.0%: Yanke 1 (naked)>Wuchuan small oat (naked)>Paul (naked)>Daoyan (hulled)>Baiyan 7 (hulled)>Zhaoshi Mountain Daoyan (naked)>Youmai 4400 (naked). These varieties can be used as the basis for breeding high β-glucan oats [22]. In addition, the location of oat β-glucan varies between varieties: in varieties with low content, it is mainly located in the aleurone layer and subaleurone layer, while in varieties with high content, it is also distributed in high concentrations in the endosperm [23]. Oat bran is a by-product of the oat flour processing process, and is mainly composed of the outermost layer of hulled oats and some endosperm. Studies have shown that the β-glucan content of oat bran after processing is 6.6% to 11.3%, and in peeled oat flour it is 3.0% to 5.4% [24], so it is often extracted from oat bran.

2.2 Oat growing environment
The β-glucan content of the same species of oats grown in different years and regions varies greatly, indicating that environmental factors such as rainfall, temperature, and soil quality have a significant impact on the formation and accumulation of oat β-glucan. The temperature during grain maturity is relatively high, and the β-glucan content of the grains is also higher. Low rainfall, drought or water stress can also lead to an increase in β-glucan accumulation in the grain. Conversely, in areas with a mild climate and high precipitation, β-glucan content is often low [25]. Yi Ying et al. studied the β-glucan content of four oat genotypes, Damou No. 1, Damou No. 2, Damou No. 3 and Damou No. 10, in Shenyang, Tai'an and the four experimental sites in the Dashang area, the place of introduction, the β-glucan content and its relationship with meteorological factors. The results showed that higher temperatures during growth and development are not conducive to the accumulation of β-glucan content, while longer hours of sunshine are conducive to an increase in β-glucan content, indicating that oats produced in areas with low temperatures and strong sunshine have higher β-glucan content [26].
2.3 Effect of processing
Studies have found that different processing techniques can cause changes in the β-glucan content and various physicochemical properties of oats, including viscosity, fluidity, molecular weight, and chemical structure. A study by Liu Wensheng et al. [27] showed that after infrared roasting of oat grains, the β-glucan content in oat flour did not change significantly; however, after being stir-fried and steamed, it was 0.76% higher than the control group on average. Roasting, steaming and infrared baking all lower the gelatinization temperature of oat flour and increase peak viscosity, final viscosity and trough viscosity.
After baking, the extraction rate of β-glucan from oat flour increases, and the ratio of trimers and tetramers in the extracted β-glucan also increases [28]. while the proportion of high molecular weight (MW>1×106) β-glucan decreased, the proportion of low molecular weight increased, and the β-glucan with a molecular weight between 1×106 and 2×106 decreased by nearly 50% [29].
The high temperature, pressure and shear forces during extrusion may cause the breaking of intermolecular bonds, molecular fragmentation and changes in molecular polarity, which in turn leads to the β-glucan in the product being more prone to aggregate. The gelation temperature, solubility, swelling degree, apparent viscosity and consistency coefficient all increase, while the flow behavior index decreases [30].
After homogenization, especially high-pressure homogenization, the mechanical cleavage of β-glucan increases its structural storage stability, which in turn leads to an increase in solubility. After homogenization, the viscosity of the solution decreases significantly, and the fluid properties change from shear thinning to Newtonian fluidity [32].
During the oxidation process, β-glucan is degraded and its viscosity decreases. The oxidation of β-glucan increases the number of carbonyl and carboxyl groups, which changes the swelling capacity of the molecule and enhances its ability to bind bile acids [33]. During germination, the overall content of β-glucan in oats tends to decrease significantly. Milling has no effect on the structure of β-glucan, but it does affect the molecular weight of β-glucan in oats, which in turn leads to differences in viscosity [34]. Edible fungi such as Ganoderma lucidum and Agaricus blazei Murill have a strong degrading effect on oat β-glucan [35].
2.4 Effect of extraction process conditions
Pan Yan et al. [36] optimized the extraction of oat β-glucan by water extraction method, and concluded that the optimal conditions for extracting oat β-glucan by water extraction method are: pH 12, liquid-to-material ratio of 25 mL/g, temperature of 40 °C, the operation time was 4 h. Qiao Youming [13] and others used gel chromatography to analyze the molecular weight of oat β-glucan extracted by water extraction with different extraction factors. It was found that the relative molecular mass distribution of oat β-glucan products ranged from 3.64×104 to 1.67×106. Under the conditions of an enzyme-inactivating temperature of 140 °C, the degreasing time was 10 min, the extraction temperature was 80 ℃, the extraction time was 1 h, the pH was 11, and the liquid-to-material ratio was 12 mL/g.
Under these conditions, the relative molecular mass of the oat β-glucan obtained was larger. Conversely, at an endonuclease inactivation temperature of 60 ℃, a degreasing time of 50 min, an extraction temperature of 40 ℃, the extraction time was 2 h, the pH was 1 or 7, and the liquid-to-material ratio was 20 mL/g. The relative molecular mass of oat β-glucan was minimal. Li Xiaopeng et al. [37] compared and preliminarily studied the molecular weight and transdermal absorption rate of oat β-glucan extracted by water, enzyme and fermentation. The results showed that the molecular weight of β-glucan was: water extraction method>enzyme extraction method>fermentation method; the skin penetration rate was: fermentation method>enzyme extraction method>water extraction method. This conclusion has a certain guiding significance for the selection of the extraction process in the industrial production of oat β-glucan.
3 Summary
Through a summary of the published data, we found that current research on the structure, properties and preparation methods of β-glucan powder at home and abroad has been quite in-depth, but there are still deficiencies in the discussion of the mechanism of action of its physiological functions.
The content, distribution and molecular weight of oat β-glucan: the content of naked oats is higher than that of oats with bran, and the proportion of soluble β-glucan is also higher, which is more beneficial for cosmetic applications; the genotype of oats also has a significant effect on the content and distribution of β-glucan. In varieties with low content, β-glucan is mainly distributed in the aleurone layer and subaleurone layer, and the extraction rate from oat bran is relatively high; while in varieties with high content, β-glucan is also distributed at a high concentration in the endosperm cells.

For different pretreatment processes, roasting, steaming, baking, and extrusion are beneficial for improving the extraction rate of oat β-glucan. After baking, the molecular weight of oat β-glucan will become relatively small, which is more conducive to absorption and utilization. Roasting, steaming, infrared baking, extrusion, homogenization will lead to an increase in the viscosity of oat β-glucan; homogenization is also conducive to an increase in solubility; oxidation treatment will lead to changes in the chemical structure of oat β-glucan; some fungi will also degrade oat β-glucan. The water extraction method is widely used in the extraction process of oat β-glucan, and the research on extraction conditions has been relatively well established. The molecular weight of the fermentation method is smaller, the skin penetration rate is higher, and the effect is better. It is worth further optimizing and improving the process.
In addition, the research on the differences in molecular weight, branching degree, and spatial geometric conformation of oat β-glucan obtained by different pretreatment and preparation processes has relatively fragmented conclusions, and no systematic analysis has been reported. What are the special properties of β-glucan with specific molecular weight, branching degree and spatial geometric conformation in terms of viscosity, fluidity and biological activity? What processing should be done to make it more suitable for absorption by the human absorption system and to maximize its efficacy? These questions also require further research.
References:
[1] Cao Min, Chen Jun, Wang Yuanchun, et al. Research progress of dextran [J]. Guangxi Light Industry, 2011(4): 17-20
[2] E. J. Wandam, translated by Chen Daijie. Biological Macromolecules, Vol. 5, Polysaccharides I: Prokaryotic Polysaccharides [M]. Beijing: Chemical Industry Press, 2004
[3] Saito H,Yoshioka Y,Uehara N,et al.Relationship between conforma- tion and biological and biological response for(1→3)-β-D-glucans in the activation of coagulation factor G from limulus amebocyte lysate and host mediated antitumor activity:Demonstration of single- helix conformation as stimulant [J]. Carbohydrate Research , 1991(217):181-190
[4] Cai Chenggang, Jiang Xinlong, Jiang Changhai, et al. Research progress on the structure, function and development of β-glucan [J]. Agro-Products Processing, 2011, 9(9): 114-117
[5] Zhang Hua, Fang Rejun. β-glucan's immune enhancement and growth promotion effects on animals and their mechanisms [J]. Hunan Feed, 2009(4):29- 31
[6] Brown G D, Gordon S. Immune recognition. A new receptor for beta- glucans[J]. Nature, 2001,413:36-37
[7] Ma X, Lei H, Li Q, et al. Molecular composition and hypoglycemic effect of the polysaccharide from the fruiting body of Grifola frondosa[J]. Pharmaceutical Biotechnology, 2007, 14(5):328-333
[8] Ning Hongzhen, Liu Yingli, Tang Yongmei, et al. The effect of oat beta-glucan combined with VC on lipid metabolism and antioxidant function in rats with hyperlipidemia [C]. Nutrition and Chronic Diseases – Proceedings of the First Academic Exchange Meeting of the Youth Working Committee of the 7th Council of the Chinese Nutrition Society, 2010
[9] Yan Mingqiang. Application of β-glucan in cosmetics [J]. Perfume, Essence and Cosmetics, 2007, 12(6):31-34
[10] Xiong Yan, Han Xiaofan, Luo Jincai. The important function of vascular endothelial growth factor in non-angiogenesis [J]. Advances in Physiological Sciences, 2011, 42(1):6-8
[11] Wang M, Ding X. Relationship between the biological activity and structure of dextran. Journal of Wuxi University of Light Industry, 1997(2):90-94
[12] Yang W, Wu H, Lai F, et al. Research progress on the physical properties and physiological functions of oat β-glucan. Modern Food Science and Technology, 2007, 23(8):90-93
[13] Qiao Youming, Duan Zhonghua, Zhu Haimei, et al. The effect of extraction factors on the relative molecular mass of oat β-glucan [J]. Food Science and Technology, 2009, 34(2): 172-176
[14] Wang Haibo, Xu Qunying, Liu Dachuan, et al. Study on the rheological properties of oat β-glucan [J]. Transactions of the Chinese Society of Agricultural Engineering, 2008, 24(5): 31-36
[15] Wang Fengmei, Fan Mingshou, Zheng Kekuan. Health benefits of oat β-glucan and factors affecting its accumulation [J]. Journal of Cereal Science, 2005, 25(2): 116-118
[16] Peterson D M,Qureshi A A.Genotype and environment effects on to - cols of barley and oats[J].Cereal Chemistry,1993,70(2):157-162
[17] Autiok, Myllymakio. Flow properties of solution of oatβ-glucan[J]. Food science, 1987,52(5):564-568
[18] Wang Xiaolei, Zhao Xiaowan, Si Huiqing. Research progress and application of oat β-glucan in food [J]. Grain and Feed Industry, 2011(3):42-43
[19] Ma Yazhen, Gao Ruiping, Cui Jun, et al. In vitro study on the adsorption of EGCG by oat β-glucan in the digestive tract [J]. Food and Fermentation Industry, 2011, 59(19):10737-10746
[20] Li Zhen, Fan Mingshou. Research on the accumulation law of B-glucan in oat grains [D]. Hohhot: Inner Mongolia Agricultural University, 2007: 5
[21] Doehlert D C,McMullen M S,Hammond J.J.Genotypic and environ - mental effects on grain yield and quality of oat grown in North Dako - ta[J]. Crop Sci,2001,41:1066-1072
[22] Zhang Haifang, Zhao Liqin, Su Xiaoyan, et al. Effect of variety and region on the β-glucan content of oats [J]. Grain and Feed Industry, 2013 (8): 34-36
[23] Miller S S,Fulcher R G.Distribution of (1-3),(1-4)-beta-D-Glucan in Kernels of Oats and Barley Using Micro spectrofluorometry [J]. Cereal Chem,1994,71:64-68
[24] Zhang Meili, Gao Julin, Wuhang Qimu, et al. A comparative study of the characteristics of β-glucan from naked oat bran and edible gum [J]. Food and Fermentation Industry, 2006, 32(8): 44-47
[25] Deng Wanhe, Wang Qiang, Lv Yaochang, et al. Effect of variety and environmental effects on the content of oat β-glucan [J]. Chinese Journal of Cereals, Oils and Foodstuffs, 2005, 20(2): 30-32
[26] Yi Ying, Qi Hua, Jin Lulu, et al. Effect of genotype and environment on β-glucan content in oats [J]. Small Grains Crops, 2009, 29(5): 333-336 [27] Liu Wensheng, Hu Xinzhong. Effect of different enzyme-inactivating treatments on the quality of oat flour [J]. Journal of Cereal Crops, 2010, 30(3): 564-567
[28] Shank Lingke, Chen Hongbing, Gao Jinyan, et al. Research progress on the effect of processing on oat β-glucan [J]. Food Industry Science and Technology, 2012(20): 366-369
[29] Uma Tiwari ,Enda Cummins,Nigel Brunton.A Modelling Approach to Estimate the Level and Molecular Weight Distribution of β-Glucan During the Baking of an Oat -Based Bread [J].Food Bioprocess Technol, 2012(1):1990-2002
[30] ZHANG M,BAI X,ZHANG Z S. Extrusion process improves the functionality of soluble dietary fiber in oat bran[J]. Journal of Cereal Science,2011,54:98-103
[31] CAMIRE M E,FLINT S I. Thermal processing effects on dietary fiber composition and hydration capacity in corn meal,oat meal,and potato peels[J]. Cereal Chem,1991,68:645-647
[32] KIVELA R,PITKANEN L,LAINE P,et al.Influence of homogenisa - tion on the solution properties of oat β-glucan[J]. Food Hydrocol - loids,2010,24:611-618
[33] MOURA F A D, PEREIRA J M. Effects of oxidative treatment on the physicochemical,rheological and functional properties of oat β-glu- can[J]. Food Chemistry,2011,128:982-987
[34] WIKSTROM K,LINDAHL L. Rheological studies of water-soluble (1→3),(1→4)-β-D-glucans from milling fractions of oat[J]. Food Science,1994,59:1077-1080
[35] Zhang Zhe, Shi Junling. Research on liquid fermentation of edible fungi and fermented beverages of oats [J]. Food Science, 2010, 31(5): 169-174
[36] Pan Yan, Wu Hao. Orthogonal optimization of oat β-glucan extraction and its molecular characterization [J]. Journal of Beijing University of Commerce and Industry (Natural Science Edition), 2009, 27(5): 5-9
[37] Li Xiaopeng, Suning, Wang Changtao, et al. Molecular weight and transdermal absorption of oat β-glucan extracted by different methods [J]. Food Science and Technology, 2011, 36(12): 252-256


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese






