Lycopene What Is It?
Lycopene is a natural carotenoid that is found mainly in ripe tomatoes, watermelons, guavas, rose hips, papayas, and grapefruits [1]. In addition, marine halophilic archaea can also produce lycopene [2]. The human body cannot synthesize lycopene on its own, and 85% of its intake comes from tomatoes and tomato-based products [3]. In the past few decades, functional research on lycopene has focused on its antioxidant, lipid-lowering, anti-inflammatory, and anti-tumor properties [4-7].
As people's awareness of food safety has gradually increased, some natural plant extracts have become the focus of research in the context of antibiotic-free farming. Lycopene has advantages in improving animal health and enhancing the quality of animal products, making it a natural plant additive with great potential [8-9]. However, at this stage, the amount added and the effect of lycopene in the breeding of different types of animals are not consistent, and the mechanism of action is not yet clear, so the degree of application and promotion is not high. In this paper, the physical and chemical properties, safety, biological functions and application progress of lycopene in animal production are reviewed, with a view to providing a reference for further research and utilization of lycopene.
1 Physical and chemical properties and safety of lycopene
1.1 Physical and chemical properties
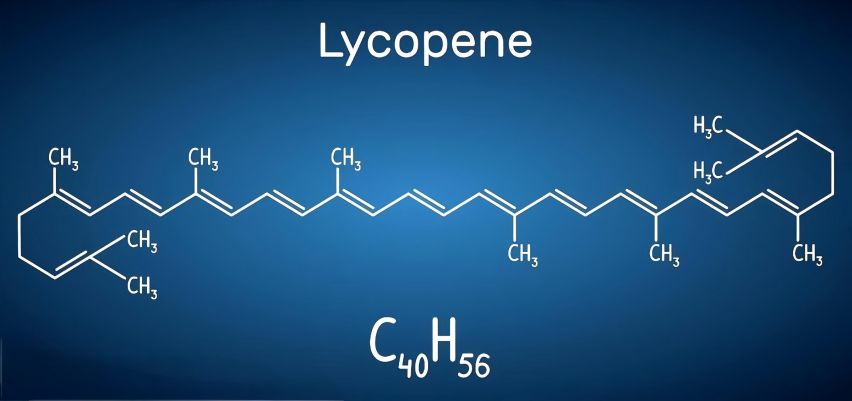
Lycopene is a fat-soluble carotenoid that belongs to the isoprene unsaturated alkenes. It has 11 conjugated double bonds and 2 non-conjugated double bonds, with a molecular formula of C40H56 and a relative molecular mass of 536.85. It has a melting point of 172–175°C and is a dark red powder. It is soluble in chloroform, hexane, benzene, carbon disulfide, acetone, petroleum ether and petroleum, insoluble in water, ethanol and methanol, sensitive to light, oxygen, high temperature, acid, catalyst and metal ions [10]. Among all carotenoids, lycopene has the highest degree of unsaturation. It mainly exists in the all-trans configuration in natural fruits and vegetables, but is easily oxidized and degraded and undergoes isomerization under the influence of heat and light [11].
1.2 Safety of lycopene
Lycopene has a long history of safe consumption worldwide. Perucatti et al. [12] showed that a diet rich in lycopene does not produce any mutagenic activity and is not toxic to rabbit lymphocytes. Lycopene has low acute toxicity in mice, and 3 g/kg body weight of lycopene administered orally and intraperitoneally has no effect on mice [13]. Moreover, no significant systemic toxicity was observed in subchronic and chronic safety studies [14]. Therefore, lycopene is considered to be a safe and non-toxic substance.
2. Physiological functions of lycopene
2.1 Antioxidant function
Oxidative stress is considered to be one of the main factors leading to many diseases. Lycopene can relieve oxidative stress damage through antioxidant action, thereby improving the health of animals.
2.1.1 Direct scavenging of free radicals
Lycopene has 11 conjugated double bonds and is highly reactive with oxygen and free radicals [15]. Lycopene is the most effective antioxidant among the various common carotenoids [16], and its rate constant for singlet oxygen scavenging is twice that of β-carotene [17]. In addition, lycopene can in vitro and intracellularly scavenge peroxynitrite [18-19]. Mortensen et al. [20] showed that lycopene has the ability to scavenge nitrogen dioxide and sulfuryl and sulfuryl radicals. Galano et al. [21] reported that lycopene is more efficient than carotene in scavenging hydrogen peroxide radicals in non-polar environments.
2.1.2 Enhancing the activity of the antioxidant system
Lycopene can indirectly affect free radicals by regulating the production of antioxidant enzymes, thereby protecting the body from oxidative damage. Studies have shown that in a rat model of type 2 diabetes, lycopene significantly increased the activity of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase (CAT) [22-23]. Li Li [24] explored the intervention effect of lycopene on esophageal cancer in rats and found that after lycopene intervention, the enzyme activities of GSH-Px and SOD in rat serum increased significantly, while the level of malondialdehyde (MDA) decreased, improving the oxidative stress state of esophageal cancer rats. In addition, lycopene can increase the body's non-enzymatic antioxidant content. Adding lycopene to the diet of rabbits can significantly increase the blood levels of vitamins A and E, while significantly reducing the level of oxidants [25]. Sahin et al. [26] supplemented the diet of heat-stressed quails with lycopene and found that the serum levels of vitamin A, vitamin C and vitamin E increased, which alleviated the oxidative stress caused by heat stress in quails and reduced the consumption of antioxidants by quails.
At present, the mechanism by which lycopene reduces oxidative stress damage in organisms has been elucidated mainly through in vivo studies. Nuclear factor erythroid 2-related factor 2 (Nrf2) is an important transcription factor in the antioxidant stress response that protects cells from oxidative stress [27]. Nrf2 mediates the expression of a series of drug metabolizing and cytoprotective enzymes, such as heme oxygenase-1 (HO-1), NAD(P)H: quinone oxidoreductase 1 (NQO-1), SOD and glutathione S-transferase (GST), etc., to enhance the removal and detoxification of endogenous and exogenous oxidants (such as reactive oxygen species) [28].
Studies have shown that lycopene significantly activates the mRNA expression of the antioxidant enzymes HO-1 and NQO-1 in the hippocampus of rats, which can reduce oxidative damage by activating the Nrf2 signaling pathway [29]. Similarly, Zhao et al. [30] found that lycopene increased antioxidant capacity by mediating the Nrf2 signaling pathway, thereby suppressing oxidative stress induced by phthalic acid (2-ethylhexyl ester) in mouse mesenchymal cell damage. In summary, lycopene can directly scavenge or inhibit free radicals, and it can also act as an inducer to activate antioxidant signaling pathways, enhance the activity of the body's antioxidant system, and exert antioxidant function through various mechanisms to relieve oxidative stress damage and protect animal health.
2.2 Lipid-lowering function
Lycopene can regulate lipid metabolism and lower blood lipid levels. In a rat model with a high-fat diet, lycopene supplementation significantly reduced the levels of total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C) and oxidized low-density lipoprotein (Ox-LDL) in the serum and brain [31]. Similarly, lycopene reduced the serum TC, TG, LDL-C and liver TC and TG levels in diabetic rats [22]. In vitro studies have shown that lycopene binds to the hydrophobic part of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, competitively inhibiting HMG-CoA reductase activity [32] and thereby reducing cholesterol synthesis. In addition, research by Fenni et al. [33] showed that lycopene can reduce the gene expression of sterol regulatory element binding protein (SREBP-1c) and fatty acid synthase (FASN) in mice with diet-induced obesity, and upregulate the expression of three genes related to fatty acid oxidation. Therefore, the lipid-lowering function of lycopene may be related to its ability to inhibit the activity of the rate-limiting enzyme in cholesterol synthesis and enhance the β-oxidation of fatty acids.
2.3 Anti-inflammatory function
Lycopene has an anti-inflammatory function, which can improve liver inflammation in mice with non-alcoholic fatty liver disease [34] and reduce the level of inflammatory factors in prostate cancer cells [35]. Yang et al. [36] found that lycopene significantly reduced the expression of matrix metalloproteinase-13 (MMP-13), serum interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α) in human osteoarthritis articular chondrocyte cells, and played a protective role. The mechanism may be through activation of the Kelch-like epoxyeicosatrienoic acid-related protein-1 (Keap1)-Nrf2 signaling pathway. Similarly, Sun et al. [37] showed that lycopene reduced the expression of TNF-α, IL-6 and IL-1β in H2O2-induced bovine mammary epithelial cells. The mechanism may be through inactivation of the nuclear factor-κB (NF-κB) signaling pathway, thereby reducing the expression of pro-inflammatory cytokines.
Other studies have reported that lycopene significantly increased the serum levels of IL-2, interferon gamma (IFN-γ) and TNF-α and the mRNA expression of IL-2, IFN-γ and TNF-α in the spleen of mice exposed to aflatoxin B1 (AFB1), can alleviate AFB 1-induced immunosuppression by inhibiting oxidative stress and mitochondrial-mediated apoptosis of mouse splenocytes [38]. In summary, lycopene mainly inhibits the inflammatory response by blocking the activation of the NF-κB signaling pathway and limiting the production of inflammatory mediators such as IL and TNF-α.
2.4 Anti-cancer function
Previous studies have shown that lycopene is associated with a reduced risk of prostate, breast, stomach, lung and colon cancers [39]. Lycopene has an inhibitory effect on the carcinogenesis of the esophageal mucosa in rats, which can be achieved by up-regulating the expression of peroxisome proliferator-activated receptor gamma (PPARγ) and cysteine-aspartic acid protease-3 (Caspase-3) protein expression, and downregulating NF-κB and cyclooxygenase-2 (COX-2) protein expression to inhibit the occurrence of esophageal cancer. The medium-dose lycopene intervention group (25 mg/kg body weight) had the best inhibitory effect on esophageal cancer [24]. In addition, in a model of lung cancer induced by the tobacco carcinogen NNK in ferrets, lycopene was found to prevent lung cancer cell proliferation by inhibiting the expression of the lung α7 nicotinic acetylcholine receptor and its downstream proteins [40]. Although current research on the anti-cancer properties of lycopene is very promising, there is a general lack of research on the mechanism of action in the clinical setting, which still needs to be explored in greater depth by scientific researchers.
2.5 Inhibits osteoporosis
Studies have shown that the higher the lycopene level in the serum of Chinese women, the higher their bone mineral density [41]. At the cellular level, lycopene has been shown to inhibit the differentiation of osteoclasts and stimulate the proliferation and differentiation of osteoblasts [42]. The mechanism may be the direct effect of lycopene on the mitogen-activated extracellular signal-regulated kinase (MEK) and protein kinase C (PKC) pathways during osteoclast formation and on MEK and NF-κB during osteoblast formation [43].
3 Application of lycopene in animal production
3.1 Application of lycopene in poultry production
Lycopene powder can improve poultry performance, improve egg quality, reduce heat stress damage, and improve reproductive performance. Sun et al. [44] found that adding 40 mg/kg lycopene to the diet of laying hens can significantly increase the initial weight and antioxidant capacity of broiler chicks. Adding lycopene (10 and 20 mg/kg) to the feed of laying hens can increase the lycopene content in the liver and egg yolk, while reducing the MDA content in the serum and eggs stored for 4 weeks [45]. Studies have shown that adding 200 mg/kg lycopene to the feed can reduce the serum TC and TG content of laying hens, as well as the cholesterol content of the liver, egg yolk, and breast muscle [46].
Sahin et al. [26] added 50, 100, and 200 mg/kg lycopene to the diets of quails under heat stress (34 °C), and found that it could improve the feed conversion rate and carcass weight of heat-stressed quails. Subsequent research by Sahin et al. [47] showed that the addition of lycopene (200 and 400 mg/kg) to the feed can alleviate the symptoms of heat stress in broilers, as lycopene increases the expression of the Nrf2 gene in the muscle and the activity of antioxidant enzymes (SOD, GSH-Px) in the serum. Najafi et al. [48] showed that lycopene can improve the total motility, membrane integrity and mitochondrial activity of frozen-thawed rooster sperm, as well as the hatchability of fertilized eggs from artificial insemination. At present, the additive amount of lycopene in poultry feed ranges from a few tens to a few hundreds of milligrams, and the experimental groups are small, so further research is needed to determine the optimal additive amount in large groups.

3.2 Lycopene in ruminant production
Adding lycopene powder to the feed can improve the growth and development and meat quality of Bamei mutton sheep in hot summer environments [49]. Xu et al. [50] showed that the addition of 200 mg/kg and 400 mg/kg lycopene to the feed of lake sheep could increase the colour stability and the content of antioxidant substances (vitamin A and vitamin E) in the stored mutton, and reduce the degree of lipid and protein oxidation. In addition, lycopene can significantly improve the mitochondrial activity of bull sperm after thawing [51], and can also improve the quality of cattle embryos cultured in vitro [52]. Ren et al. [53] showed that the addition of 1.0 mg/mL lycopene to the cryopreserved cashmere goat semen significantly improved sperm motility, acrosome integrity, mitochondrial activity, and the activity of antioxidant enzymes in goat sperm.
The group with 4.0 mg/mL lycopene did not improve the physiological characteristics of the sperm. Tvrdá et al. [54] showed that In a model of oxidative stress damage induced by ferrous ascorbate in bovine sperm, the addition of lycopene (0.25, 0.50, 1.00, 2.00 mmol/L) can improve sperm activity and significantly inhibit the increase in MDA in the sperm suspension. The most effective lycopene concentrations are 1.00 and 2.00 mmol/L. The mechanism may be that lycopene can easily pass through biological membranes and quickly enter cells, playing an important role in protecting cell membranes and lipoproteins from oxidative damage. The above results show that lycopene has the effect of improving the production performance of ruminants, improving the quality of stored lamb, and improving the quality of sperm from breeding animals. In order to achieve the best application results, attention should be paid to the amount of additive during the application process.

3.3 Application of lycopene in pig production
There are few reports on the use of lycopene in pig production. Wang Jie [55] showed that adding a certain concentration of lycopene to the frozen dilution of pig sperm can significantly improve the quality of the sperm after thawing, and the most suitable addition level is 2.0 μmol/L. Studies have shown that adding 20 mg/kg lycopene to the pig feed can reduce the MDA content of fresh pork [56]. Adding lycopene (12.5, 25.0, 37.5, 50.0 mg/kg) to the feed of fattening pigs can reduce the MDA content of pork during storage (0, 24, 48, 72 h), with the best effect being achieved when the lycopene addition is 50.0 mg/kg [57]. The mechanism may be to increase the lycopene content in the meat, thereby improving the oxidative stability of the pork. In addition, Correia et al. [58] found that adding 5% tomato pomace to piglets' feed can also increase the content of α-tocopherol in muscles and livers and improve the quality of pork.
3.4 Lycopene in aquatic animals
Lycopene has the effect of improving the production performance of aquatic animals and reducing oxidative stress damage. Adding 0.2% lycopene to a high-fat diet can improve the feed conversion efficiency, protein efficiency ratio and antioxidant function of rainbow trout, and the effect is better than that of the antioxidant ethoxyquin [59]. Studies have shown that lycopene can reduce the toxic effects of malathion on carp [60] and carbofuran on African catfish [61]. The mechanism may be that lycopene reduces the MDA content and increases the activity of antioxidant enzymes by scavenging free radicals. These findings provide a theoretical basis for lycopene to reduce the damage caused by drugs in aquatic animals. However, some studies have shown that lycopene can reduce the activities of SOD, CAT and GSH-Px in the liver of golden perch. The mechanism may be that lycopene can effectively scavenge free radicals and prevent oxidative stress, so it stimulates endogenous antioxidant enzymes less [62].

3.5 Application of lycopene in rabbit production
Lycopene has the effect of improving the antioxidant capacity of meat rabbits, improving meat quality, and lowering cholesterol. Lorenz et al. [63] fed New Zealand white rabbits a high cholesterol diet for 4 weeks, while supplementing with lycopene (5 mg/kg body weight). The results showed that the lycopene level in rabbit plasma was significantly increased, and the serum TC and LDL-C concentrations were reduced by nearly 50%. Studies have shown that adding 5 mg/kg lycopene to rabbit feed can increase the levels of retinol and α-tocopherol in rabbit meat, while also reducing the cholesterol content of rabbit meat and the thiobarbituric acid reactive substances (TBARS) content of rabbit meat after refrigeration for 3 d [64-65]. In addition, the addition of lycopene to the feed can reduce the TBARS content of the plasma of rabbits and increase the content of vitamin A and α-tocopherol [65]. The mechanism may be that lycopene can reduce the production of free radicals and increase the activity of antioxidant enzymes.

4 Summary
In summary, lycopene has important physiological functions, can improve animal production performance, reduce blood lipids, improve antioxidant capacity and reproductive performance, and is currently being studied more and more in animal production. In the production of ruminants, poultry, rabbits and other animals, lycopene has shown the potential to improve the quality of animal products, which provides a basis for the research and development of healthy livestock products. Therefore, lycopene has broad application prospects as a feed additive. China is a major tomato-producing country, and in-depth development of the functions of lycopene is not only beneficial to the healthy development of the breeding industry, but also to the deep processing of agricultural products. However, at this stage, research on the appropriate addition level and mechanism of lycopene in animal production is still lacking, and the degree of application and promotion is not high. Therefore, under the general trend of “antibiotic-free farming,” there is an urgent need to further study the appropriate addition levels, effects, and regulatory mechanisms of lycopene in various animals, in order to provide a theoretical basis for the better application of lycopene in animal husbandry.
Reference:
[1]Saini R K,A Bekhit AE, Roohinejad S, et al. Chemical stability of lycopene in processed products: A review of the effects of processing methods and modern preservation strategies[J]. J Agric Food Chem, 2020, 68(3): 712-726.
[2]Rodrigo-Baños M, Garbayo I, Vílchez C, et al. Carotenoids from Haloarchaea and their potential in biotechnology[J]. Mar Drugs, 2015, 13: 5508-5532.
[3]Bramley P M. Is lycopene beneficial to human health[J] . Phytochem, 2000, 54(3): 233-236.
[4]Stahl W, Junghans A, de Boer B, et al. Carotenoid mixtures protect multilamellar liposomes against oxidative damage: synergistic effects of lycopene and lutein[J]. FEBS Lett, 1998, 427: 305-308.
[5]Zeng Z, He W, Jia Z, et al. Lycopene improves insulin sensiti- vitythrough inhibition of STAT3/Srebp-1c-mediated lipid accumulation and inflammation in mice fed a high-fat diet[J]. Exp Clin Endocrinol Diabetes, 2017, 125(9): 610-617.
[6]Kawata A, Murakami Y, Suzuki S, et al. Anti-inflammatory activity of β-carotene, lycopene and tri-n-butylborane, a scavenger of reactive oxygen species[J]. In Vivo, 2018, 32(2): 255-264.
[7]Jhou BY, Song TY, Lee I, et al. Lycopene inhibits metastasis of human liver adenocarcinoma SK Hep 1 cells by downregulation of NADPH oxidase 4 protein expression[J] . J Agric Food Chem, 2017, 65: 6893-6903.
[8]Garavaglia L, Galletti S, Tedesco D. Silymarin and lycopene administration in periparturient dairy cows: effects on milk production and oxidative status[J]. N Z Vet J, 2015, 63(6): 313- 318.
[9]Domínguez R, Gullón P, Pateiro M, et al. Tomato as potential source of natural additives for meat industry. A Review[J] . Antioxidants (Basel), 2020, 9(1): 73.
[10]Shi J, Le Maguer M, Bryan M. Lycopene from tomatoes. In Functional Foods-Biochemical and Processing Aspects[M] . Boca Raton: CRC Press, 2002: 135-168.
[11]Shi J, Maguer M L E, Bryan M, et al. Kinetics of lycopene degradation in tomato puree by heat and light irradiation[J]. J Food Process Eng, 2003, 25(6): 485-498.
[12]PerucattiA, Genualdo V, Pauciullo A, et al. Cytogenetic tests reveal no toxicity in lymphocytes of rabbit (Oryctolagus cuniculus, 2n=44) feed in presence of verbascoside and/or lycopene[J]. Food Chem Toxicol, 2018, 114: 311-315.
[13]Milani C, Maccari M, Mosconi P. Action of lycopene in the experimental gastric ulcer[J]. Pharmacology, 1970, 4(6): 334- 340.
[14]Michael McClain R, Bausch J. Summary of safety studies conducted with synthetic lycopene[J]. Regul Toxicol Pharm- acol, 2003, 37(2): 274-285.
[15]Krinsky N I. The antioxidant and biological properties of the carotenoids[J]. Ann NY Acad Sci, 1998, 854: 443-447.
[16]Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher[J] . Arch Biochem Biophys, 1989, 274(2): 532-538.
[17]Conn P F, Schalch W, Truscott T G. The singlet oxygen and carotenoid interaction[J]. J Photochem Photobiol B, 1991, 11: 41-47.
[18]Panasenko O M, Sharov V S, Briviba K, et al. Interaction of peroxynitrite with carotenoids in human low density lipoproteins[J]. Arch Biochem Biophys, 2000, 373: 302-305.
[19]Muzandu K, Ishizuka M, Sakamoto K Q, et al. Effect of lycopene and beta-carotene on peroxynitrite-mediated cellular modifications[J]. ToxicolApplPharmacol, 2006, 215: 330-340.
[20] Mortensen A, Skibsted L H. Relative stability of carotenoid radical cations and homologue tocopheroxyl radicals. A real time kinetic study of antioxidant hierarchy[J]. FEBS Lett, 1997, 417: 91-97.
[21]Galano A, Francisco-Marquez M. Reactions of OOH radical with beta-carotene, lycopene, and torulene: hydrogen atom transfer and adduct formation mechanisms[J]. J Phys Chem B, 2009, 113: 11338-11345.
[22]Yin Y, Zheng Z, Jiang Z. Effects of lycopene on metabolism of glycolipid in type 2 diabetic rats[J]. Biomed Pharmacother, 2019, 109: 2070-2077.
[23]Zheng Z, Yin Y, Lu R, et al. Lycopene ameliorated oxidative stress and inflammation in type 2 diabetic rats[J]. J Food Sci, 2019, 84(5): 1194-1200.
[24] Li L. Research on the effect and mechanism of lycopene intervention on the occurrence and development of esophageal cancer based on PPARγ [D]. Zhengzhou: Zhengzhou University, 2019.
[25]Corbi G, Conti V, Komici K, et al. Phenolic plant extracts induce Sirt1 activity and increase antioxidant levels in the rabbit's heart and liver[J]. Oxid Med Cell Longev, 2018, 2018: 2731289.
[26]Sahin K, Onderci M, Sahin N, et al. Effects of lycopene supplementation on antioxidant status, oxidative stress, perfor- mance and carcass characteristics in heat-stressed Japanese quail[J]. J Therm Biol, 2006, 31(4): 307-312.
[27]Suzuki T, Yamamoto M. Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress[J]. J Biol Chem, 2017, 292(41): 16817-16824.
[28]Ma Q. Role of Nrf2 in oxidative stress and toxicity[J]. Annu Rev Pharmacol Toxicol, 2013, 53: 401-426.
[29]Zhao B, Ren B, Guo R, et al. Supplementation of lycopene attenuates oxidative stress induced neuroinflammation and cognitive impairment via Nrf2/NF-κB transcriptional pathway[J]. Food Chem Toxicol, 2017, 109: 505-516.
[30]Zhao Y, Li M Z, Shen Y, et al. Lycopene prevents DEHP- induced Leydig cell damage with theNrf2 antioxidant signaling pathway in mice[J] . J Agric Food Chem, 2020, 68(7): 2031- 2040.
[31]Yang W, Shen Z, Wen S, et al. Mechanisms of multiple neurotransmitters in the effects of lycopene on brain injury induced by Hyperlipidemia[J]. Lipids Health Dis, 2018, 17(1): 13.
[32]Alvi S S, Iqbal D, Ahmad S, et al. Molecular rationale delinea- ting the role of lycopene as a potent HMG-CoA reductase inhibitor: In vitro and in silico study[J]. Nat Prod Res, 2016, 30: 2111-2114.
[33]Fenni S, Hammou H, Astier J, et al. Lycopene and tomato powder supplementation similarly inhibit high-fat diet induced obesity, inflammatory response, and associated metabolic disorders[J]. Mol Nutr Food Res, 2017, 61: 1601083.
[34]Ni Y H, Zhuge F, Nagashimada M, et al. Lycopene prevents the progression of lipotoxicity-induced nonalcoholic steatohepatitis by decreasing oxidative stress in mice[J]. Free Radic Biol Med, 2020, 152: 571-582.
[35]Jiang LN, Liu Y B, Li B H. Lycopene exerts anti-inflammatory effect to inhibit prostate cancer progression[J]. Asian J Androl, 2019, 21: 80-85.
[36]Yang J J, Song X B, Feng Y, et al. Natural ingredients-derived antioxidants attenuate H2O2-induced oxidative stress and have chondroprotective effects on humanosteoarthritic chondrocytes via Keap1/Nrf2 pathway[J]. Free Radic Biol Med, 2020, 152: 854-864.
[37]Sun X, Jia H, Xu Q, et al. Lycopene alleviates H2O2-induced oxidative stress, inflammation and apoptosis in bovine mam- mary epithelial cells via the NFE2L2 signaling pathway[J] . Food Funct, 2019, 10(10): 6276-6285.
[38]Xu F, Wang P, Yao Q, et al. Lycopene alleviates AFB1-induced immunosuppression by inhibiting oxidative stress and apoptosis in the spleen of mice[J]. Food Funct, 2019, 10(7): 3868-3879.
[39]Rowles J L, Erdman J W. Carotenoids and their role in cancer prevention[J] . Biochim Biophys Acta Mol Cell Biol Lipids, 2020,1865(11): 158613.
[40]Aizawa K, Liu C, Tang S, et al. Tobacco carcinogen induces both lung cancer and non-alcoholic steatohepatitis and hepatocellular carcinomas in ferrets which can be attenuated by lycopene supplementation[J]. Int J Cancer, 2016, 139(5): 1171-
1181.
[41]Zhang Z Q, Cao W T, Liu J, et al. Greater serum carotenoid concentration associated with higher bone mineral density in Chinese adults[J]. Osteoporos Int, 2016, 27(4), 1593-1601.
[42]Rao L G, Krishnadev N, Banasikowska K, et al. Lycopene I-effect on osteoclasts: lycopene inhibits basal and parathyroid hormone-stimulated osteo clast formation and mineral resorption mediated by reactive oxygen species in rat bone marrow cultures[J]. J Med Food, 2003, 6(2): 69-78
[43]Costa-Rodrigues J, Fernandes M H, Pinho O, et al. Modulation of human osteoclastogenesis and osteoblastogenesis by lycopene[J]. J Nutr Biochem, 2018, 57: 26-34.
[44]Sun B, Chen C, Wang W, et al. Effects of lycopene suppleme- ntation in both maternal and offspring diets on growth performance, antioxidant capacity and biochemical parameters in chicks[J]. JAnim PhysiolAnim Nutr (Berl), 2015, 99(1): 42-49.
[45]An B K, Choo W D, Kang C W, et al. Effects of dietary lycope ne or tomato paste on laying performance and serum lipids in laying hens and on malondialdehyde content in egg yolk upon storage[J]. J Poult Sci, 2019, 56(1): 52-57.
[46] He Chungong, Zhou Zhenbing. Effect of adding lycopene to the diet on the lipids of laying hens [J]. Hubei Agricultural Sciences, 2015, 54(4): 917-919, 925.
[47]Sahin K, Orhan C, Tuzcu M, et al. Lycopene activates antioxidant enzymes and nuclear transcription factor systems in heat-stressed broilers[J]. Poult Sci, 2016, 95(5): 1088-1095.
[48]NajafiA, Taheri R A, Mehdipour M, et al. Lycopene-loaded nanolipo some s improve the performance of a modified Beltsville extender broiler breeder roosters[J]. Anim Reprod Sci, 2018, 195: 168-175.
[49]Jiang H, Wang Z, Ma Y, et al. Effect of dietary lycopene supplementation on growth performance, meat quality, fatty acid profifile and meat lipid oxidation in lambs in summer conditions[J]. Small Ruminant Res, 2015, 131: 99-106.
[50]Xu C, Qu Y, Hopkins D L, et al. Dietary lycopene powder improves meat oxidative stability in Hu lambs[J]. J Sci Food Agric, 2019, 99(3): 1145-1152.
[51]Bucak M N, Ataman M B, Baş pınar N, et al. Lycopene andresveratrol improve post-thaw bull sperm parameters: sperm motility, mitochondrial activity and DNA integrity[J]. Andrologia, 2015, 47(5): 545-552.
[52]Chowdhury M M R, Choi B H, Khan I, et al. Supplementation of lycopene in maturation media improves bovine embryo quality in vitro[J]. Theriogenology, 2017, 103: 173-184.
[53]Ren F, Feng T, Dai G, et al. Lycopene and alpha-lipoic acid improve semen antioxidant enzymes activity and cashmere goat sperm function after cryopreservation[J]. Cryobiology, 2018, 84: 27-32.
[54]Tvrdá E, KováčikA, Tušimová E, et al. Antioxidant efficiency of lycopene on oxidative stress-induced damage in bovine spermatozoa[J]. J Anim Sci Biotechnol, 2016, 7(1): 50.
[55] Wang J. The effect of lycopene, sesaminol and baicalein on the cryopreservation of pig sperm [D]. Yangling: Northwest A&F University, 2017.
[56]An B K, Kim D H, Joo W D, et al. Effects of lycopene and tomato paste on oxidative stability and fatty acid composition of fresh belly meat in finishing pigs[J]. Ital J Anim Sci, 2019, 18(1): 630-635.
[57]Fachinello M R, Gasparino E, Monteiro AN T R, et al. Effects of dietary lycopene on the protection against oxidation of muscle and hepatic tissue in finishing pigs[J]. Asian-Australas J Anim Sci, 2020, 33(9): 1477-1486.
[58]Correia C S, Alfaia C M, Madeira M S, et al. Dietary inclusion of tomato pomace improves meat oxidative stability of young pigs[J]. JAnim PhysiolAnim Nutr (Berl), 2017, 101(6): 1215- 1226 .
[59] Zhang Jianwei, Zhang Xi, Tao Linli, et al. Effects of rubber seed oil-based feed supplemented with ethoxyquin and lycopene on the growth and antioxidant capacity of rainbow trout [J]. Journal of Yunnan Agricultural University (Natural Science), 2018, 33(6): 1081-1088.
[60]Yonar S M. Toxic effects of malathion in carp, Cyprinus carpio carpio: Protective role of lycopene[J]. Ecotoxicol Environ Saf, 2013, 97: 223-229.
[61]Hamed H S, Osman AG. Modulatory effect of lycopene against carbofuran toxicity in African catfish, Clarias gariepinus[J] . Fish Physiol Biochem, 2017, 43(6): 1721-1731.
[62]Abd El-Gawad E A, Wang H P, Yao H. Diet supplemented with synthetic carotenoids: effects on growth performance and biochemical and immunological parameters of Yellow Perch (Percaflavescens)[J]. Front Physiol, 2019, 10: 1056.
[63]Lorenz M, Fechner M, Kalkowski J, et al. Effects of lycopene on the initial state of atherosclerosis in New Zealand white (NZW) rabbits[J]. PLoS One, 2012, 7(1): e30808.
[64]Vizzarri F, Palazzo M, D’Alessandro A G, et al. Productive performance and meat quality traits in growing rabbit following the dietary supplementation of Lippia citriodora, Raphanus sativus and Solanum lycopersicum extracts[J]. Livest Sci, 2017,200: 53-59.
[65]Palazzo M, Schiavitto M, Cinone M, et al. Rabbit metabolic response and selected meat quality traits: Evaluation of dietary PLX®23 and LycoBeads® feed supplement[J]. JAnim Physiol Anim Nutr (Berl), 2019, 103(1): 383-394.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese





