How to Produce D Arabinose?
D Arabinitol is an important natural product that is widely found in lichen plants and some food-derived fungi. However, its natural content is low, and it requires a large amount of raw materials in traditional processes, which seriously affects its economic benefits and makes it unsuitable for large-scale production [1,2]. The chemical catalytic method uses nickel as a catalyst to undergo redox reactions, reducing arabinose to generate D-arabinitol. This is currently the most mainstream method for the industrial production of D-arabinitol [3]. Although the chemical catalytic method can achieve industrial scale in the market and the production technology is relatively mature, the production process requires the use of expensive catalysts and strict control of conditions such as high temperature (100°C) and high pressure (40-60 bar) [4]. This not only pollutes the environment but also makes it difficult to separate and purify the subsequent products, resulting in high production costs [5].
Compared with the chemical catalytic method, the production conditions required for the biological production of D-arabinitol are milder. The conversion of substances is achieved by the growth and metabolism of microorganisms, and the product obtained is easy to separate and purify, and the purity and yield of D-arabinitol can be improved [6, 7], which is more in line with the concept of being green, economical and environmentally friendly. There are two main biological methods: microbial fermentation and resting cell transformation. The microbial fermentation method mainly generates D-arabinitol by converting substrates such as glycerol, xylose, lactose, glucose, and xylitol during microbial growth and metabolism [8, 9]. The resting cell transformation method mainly relies on collecting and enriching the cultured bacterial liquid to prepare resting cells, and uses various enzymes present in resting cells to convert D-arabinitol.
In summary, the methods for producing D-arabinitol each have their own characteristics, but the biological method has the advantages of being green, economical and environmentally friendly, as it achieves the conversion of substances through the normal growth and metabolism of microorganisms. In addition, the products of the biological method are easy to separate and purify, which improves the purity and yield of D-arabinitol. Although the chemical catalytic method is still the main production method, the biological method has become or is becoming a more promising alternative.
1. Current Research Status of the Biological Production of D-arabitol
High-osmotic-pressure-tolerant yeast is the main microorganism in nature that can produce D-arabitol. Under high osmotic pressure, yeast cells can synthesize and accumulate polyols to cope with dehydration stress, thereby protecting the cells from damage. In 1956, Spencer et al. found that high-osmotic-pressure-tolerant yeast could produce a variety of polyols through the fermentation of glucose, including D-arabitol [10]. In 1969, Onishi et al. discovered that glucose could be converted to xylitol under the action of microorganisms, and that D-arabinitol was an intermediate product [11, 12]. This discovery laid the theoretical foundation for the biological production of xylitol and attracted widespread attention from scholars at home and abroad.
At present, research on the production of D-arabinitol mainly focuses on strain screening and fermentation condition optimization. For example, Song Weibin et al. screened a strain of Saccharomyces cerevisiae that produces D-arabinitol from pollen, and improved the conversion rate after optimizing the fermentation conditions [12]. Li Ze et al. isolated a strain of Pichia pastoris L-84 from soil microorganisms that is resistant to high osmotic pressure and produces D-arabinitol, and carried out large-scale fermentation in a fermenter [13]. Saha et al. used a strain of Rhodotorula glutinis NRRLY-27624 obtained by screening from a wild honeycomb to optimize the fermentation conditions and finally achieve the fermentation of glucose to produce D-arabinitol, with a conversion rate of 48% [14][15].
Zhang Lili successfully isolated a high-yield strain of D-arabitol from Debaryomyces hansenii and established a kinetic model for D-arabitol fermentation [16]. Nozaki screened a strain of Rhodotorula kofmei AJ14787 that produces D-arabitol efficiently. Fermentation optimization was carried out by combining variable temperature cultivation and continuous feeding of 700 g/L glucose, and finally D-arabitol was produced by fermentation 206 g/L[17]. Cheng et al. applied adaptive evolution to Pichia pastoris strains, and the yield was increased by 72.7% compared to the wild type[18].
M Povelainen et al. constructed an engineered bacterium Bacillus subtilis ATCC31094 that produces D-arabitol, which has a conversion efficiency of up to 38% and has good application prospects[19]. Subsequent researchers have found that the genera Candida[20], Kluyveromyces[21], Kodamea[22, 23], Hansenula[24, 25] and Debaryomyces[26] are capable of producing D-arabitol[27].
2. Metabolic Pathways for the Biotechnological Production of D-arabinitol
In current research, it has been found that substrates such as glucose, xylose [28], glycerol [29, 30] and lactose [31] can be used for the production of D-arabinitol. It is worth noting that glucose and xylose rank among the top two carbon source reserves in nature, and are therefore widely regarded as the most cost-effective substrates. According to existing research reports, the metabolic pathway for producing D-arabitol from glucose mainly includes two synthetic pathways, while the possible metabolic pathway for producing D-arabitol from xylose only has one, the xylitol pathway. The three common metabolic pathways of D-arabitol are shown in Figure 1.2.
Figure 1. Metabolic pathway of Darabitol
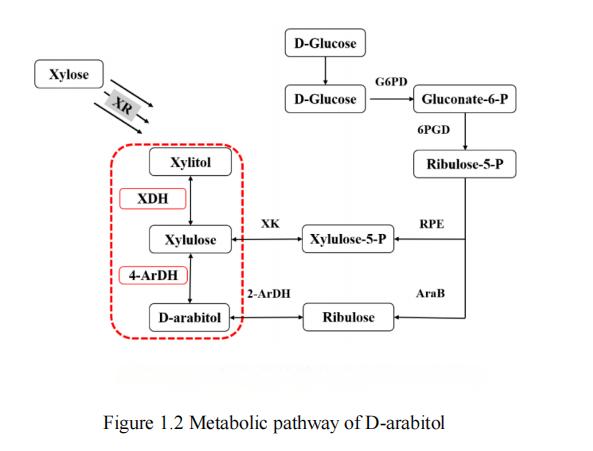
Two synthetic pathways exist for the metabolic process of producing darabitol from glucose: the ribulose pathway and the xylulose pathway. In these processes, glucose is first phosphorylated to glucose-6-phosphate, and then converted to 5-phospho-D-ribulose via the pentose phosphate pathway (PPP pathway), which is a common precursor for the ribulose and xylulose pathways. The conversion of 5-phospho-D-ribose to D-ribulose by ribulose kinase and then to D-arabinitol by 2-ArDH forms the ribulose pathway. In the other metabolic pathway, 5-phospho-D-ribose is isomerized to D-xylulose-5-phosphate and then dephosphorylated to D-xylulose. Next, D-arabitol 4-dehydrogenase converts xylulose to D-arabitol, thus forming the xylulose pathway [32].
The metabolic pathway for producing D-arabitol from xylose mainly involves a synthetic pathway, the xylitol pathway. In this process, xylose reductase first converts xylose to xylitol. Subsequently, xylitol dehydrogenase (XDH) reduces xylitol to D-xylulose. Finally, D-arabitol dehydrogenase (ArDH) converts D-xylulose to D-arabitol, thus completing the synthesis of the xylitol pathway.
In summary, glucose and xylose, as two common and inexpensive substrates in biometabolic engineering, can be used to produce D-arabinitol through various pathways such as the pentose phosphate pathway, the xylulose pathway and the xylitol pathway. These metabolic pathways provide researchers with a wealth of biosynthesis strategies, which will help to further explore and optimize the production process of D-arabinitol to meet the needs of functional sugar alcohols in practical applications.
3. Factors Affecting the Production of D-Arabinitol by Microbial Fermentation
During the fermentation process, the yield of D-arabinitol may be restricted by various factors. At present, the yield of D-arabinitol can be effectively increased by optimizing the fermentation conditions, such as pH, temperature, carbon and nitrogen source composition, inoculum size, rotation speed and dissolved oxygen concentration [33, 34]. These factors are closely related to the metabolic processes of the production host. By adjusting these parameters, ideal fermentation conditions can be established to increase fermentation yield.
At present, most research on the biological production of D-arabinitol focuses on the fermentation of D-arabinitol from glucose, and there is less research on the production of D-arabinitol from xylose. It is known that many yeasts produce D-arabinitol during glucose growth, but few yeasts are known to produce D-arabinitol from xylose. In 2018, Jagtap et al. [28] first reported that Rhodosporidium toruloides IFO0880 can produce D-arabinitol from xylose in a nitrogen-rich medium. This is the first time that it has been discovered that a natural yeast can use xylose as the sole carbon source to produce D-arabinitol, confirming the existence of a xylitol pathway in the metabolic process of biological production of D-arabinitol. In this process, xylose is first converted to xylitol by xylose reductase. Subsequently, xylitol is reduced to D-xylulose by XDH. Finally, ArDH converts D-xylulose to D-arabinitol.

A higher initial substrate concentration may exert osmotic pressure on the fermentation strain, promoting the forward bioconversion reaction and thereby increasing the yield of D-arabitol. For example, during the fermentation of Debaryomyces hansenii SBP-1, the use of 150 g/L xylose can increase the yield of D-arabitol by 2.23 times compared with the use of 70 g/L xylose. During fermentation with Debaryomyces hansenii NRRLY-7483, the use of 1.5% glycerol can increase the production of D-arabitol by 4 to 5 times compared to 0.5% glycerol [35]. During the growth and metabolism of Candida sp. H2 [36], the production of D-arabinitol increased significantly with increasing glucose concentration, with 250 g/L glucose being the optimal initial sugar concentration. However, too high a substrate concentration may also be detrimental to D-arabitol synthesis. For example, during the cultivation of Zygosaccharomyces rouxii JM-C46, the production of D-arabitol did not increase further when the glucose concentration was increased from 200 g/L to 250 g/L.
Nitrogen sources are key elements in microbial fermentation systems and are closely related to the regulation of microbial growth and metabolism. For example, the optimal nitrogen source for Candida sp. H2[37] and Candida quercitrusa is yeast extract, while ammonium sulfate is the optimal nitrogen source for Pichia Manchurica[38] and Debaryomyces Hansenii. Kumdam[39] and Loman[40] found that adding an appropriate amount of nitrogen source to the medium can increase the production of D-arabitol. Similarly, Jagtap et al. found that Rhodosporidium toruloides IFO0880 can convert xylose to produce high titers of D-arabitol in nitrogen-rich media. The yield of D-arabitol increased with the increase of nitrogen content in the medium. However, high nitrogen concentrations may be detrimental to the production of D-arabitol by Kluyveromyces ohmeri strains [41].
During microbial fermentation, the addition of the right amount of metal ions can maintain the osmotic pressure balance of the intracellular fluid, enhance the activity of intracellular enzymes, and promote microbial growth and metabolism. Yoshikawa studied the effect of metal ions on the production of D-arabinitol by Candida quercitrusa and found that calcium ions promote the production of D-arabinitol. The reason is that calcium ions penetrate into the cell and enhance the activity of enzymes in the cell growth and metabolism pathway. In another study, Kumdam [42] and Sundaramoorthy found that zinc, iron, manganese and copper ions can promote the growth metabolism of Debaryomyces nepalensis and Pichia Manchurica strains and increase the production of D-arabinitol.
3.1 Xylitol Dehydrogenase
Xylitol dehydrogenase is a key rate-limiting enzyme in the biosynthetic pathway for D-arabinitol production. XDH plays a vital role in the metabolism of xylose by microorganisms to produce D-arabinitol. As a reversible redox enzyme, the catalytic activity of XDH depends on the cofactors NAD+ and NADH [43, 44].
This enzyme is mainly found in yeasts that ferment xylose to produce xylitol, such as Paecilomyces taphios, Candida shehatae, and Pichiastipitis [45]. Filamentous fungi such as Fusarium oxysporum and Neurospora crassa are also important sources of XDH. It is worth noting that the active centre sequence of these XDHs from different species is highly conserved [46].
Masakazu et al. [47] cloned the XDH gene from Gluconobacter oxycans ATCC621, Zhang et al. [48] studied the XDH gene in Gluconobacter oxycans NH-10, and Qi et al. [49] cloned the XDH gene from Gluconobacter oxycans CGM CC 1.49.

These researchers cloned XDH genes from different strains and successfully expressed them in Escherichia coli BL21. They also studied the enzymatic properties of XDH and found that the enzymatic properties of these XDHs from different sources are similar to some extent. Further studies have found that XDH belongs to the short-chain dehydrogenase family. In the xylitol oxidation reaction with NAD+ as the coenzyme, the optimal pH of XDH is 11; while in the D-xylulose reduction reaction with NADH as the coenzyme, the optimal pH of XDH is 5. It can therefore be inferred that the optimal pH of XDH in the oxidation reaction is in the alkaline range, while the optimal pH in the reduction reaction is in the acidic range.
In summary, XDH is biologically important. The enzymatic properties of XDH in different strains vary, but they all share a certain degree of similarity in terms of protein sequence and enzymatic properties. Current research shows that XDH has different optimal pH values under different reaction conditions, which provides a basis for further research and application of the enzyme.
4 Molecular Modification
XDH has diverse catalytic properties and plays an important role in biocatalysis. However, natural enzymes still have limitations in terms of activity, substrate spectrum and catalytic specificity, which makes it difficult to achieve an ideal level of product yield. In recent years, protein engineering strategies such as directed evolution, rational design and semi-rational design have been widely used in enzyme modification. These strategies can effectively improve the performance of enzymes to better meet actual production needs.
5 Directed Evolution
Since Frances H. Arnold first proposed the concept of directed evolution in 1993, this field has made remarkable progress in recent decades [50]. The basic idea of directed evolution, which plays an important role in enzyme engineering, is to simulate the natural evolution process, introduce artificial random mutations, and screen for high-performance mutants.
In addition, the directed evolution strategy has the advantage that it can effectively modify the function of enzymes without a deep understanding of their structure, function and catalytic mechanism. The main steps of the directed evolution strategy include the construction of mutants and the screening of mutant libraries. This process uses random mutation and genetic recombination techniques such as error-prone PCR and DNA shuffling [51].
The application of these techniques has greatly improved the efficiency of mutant construction and the diversity of mutant libraries. However, the implementation of directed evolution strategies faces some challenges, especially in the high-throughput screening stage [52]. High-throughput screening, as a method for quickly identifying and selecting superior mutants, is critical to the directed evolution process [53].
The high-throughput screening method for a specific mutant library needs to be specifically designed according to the target product, so there is no universal screening method. The specific design and challenges of the screening process at this stage undoubtedly add to the complexity of the directed evolution strategy. Overall, despite the specific design and challenges of the high-throughput screening method, directed evolution technology is still considered an effective tool for molecular engineering of enzymes. Its significant contributions and continued development trends indicate that this field will continue to play an important role in biotechnology and related fields.
Reference:
[1]Escalante J, Caminal G, Figueredo M, et al. Production of arabitol from gl ucose by Hansenula polymorpha[J]. Journal of Fermentation and Bioenginee ring, 1990,70(4):228-231.
[2]Moran J W, Witter L D. Effect of sugars onDarabitol production and glucose metabolism in saccharomyces rouxii[J]. Journal of Bacteriology, 1979,138(3):823- 831.
[3]Murthy S N, Kumdam H, Gummadi SN. Arabitol production by microbial fermen- tation-biosynthesis and future applications[J]. International Journal of Sciences Basic & Applied Research, 2014, 1:1-12.
[4]Kumdam H, Narayana M S, Gummadi S N. Production of ethanol and arabitol by Debaryomyces nepalensis: influence of process parameters[J]. AMB Express, 2013,3(1):23.
[5]Zhang G, LinY, He P, et al. Characterization of the sugar alcohol-productingyeast Pichia anomala[J]. Journal of Industrial Microbiology & Biotechnology, 2014,41(1):41-48.
[6] Huang Wei, Wang Xiaodan, Li Dounan, et al. Research progress on microbial fermentation of D-arabitol [J]. China Brewing, 2017, 36(09): 6-10.
[7] Ding Xiaobing, Li Zongwei, Yang Zhuanqin, et al. Improved paper chromatography for determining the content of isoleucine in enzyme-producing liquid [J]. Food Industry Science and Technology, 2009, 30(08): 314-315.
[8] Akinosho H, Rydzak T, Borole A, et al. Toxicological challenges to microbial bioethanol production and strategies for improved tolerance [J]. Ecotoxicology, 2015, 24(10):2156-2174.
[9] Qian Weidong, Ning Xiaoxiao, Wang Lan, et al. Rapid screening method for high-yield D-arabinitol yeast strains [J]. Journal of Shaanxi University of Science and Technology, 2014(6):129-133.
[10] Spencer J F T, Sallans H R. Production of polyhydric alcohols by osmophilic yeasts [J]. Canadian Journal of Microbiology, 1956, 2(2):72-79.
[11] Onishi H, Suzuki T. Microbial production of xylitol from glucose [J]. Applied Microbiology, 1970, 18(6):1031-1035.
[12] Song Weibin, Lin Yanqing, Hu Haiyan, et al. Screening and identification of D-arabitol-producing strains and optimization of D-arabitol production conditions [J]. Acta Microbiologica Sinica, 2011, 51(03): 332-339.
[13] Li Ze, Zhao Xiangying, Liu Jianjun. Screening and identification of yeast strains producing D-arabinol [J]. Food Industry, 2012, 33(10): 27-30.
[14]Guo Q, ZabedH, Zhang H, et al. Optimization of fermentation medium for a newly isolated yeast strain (Zygosaccharomyces rouxii JM-C46) and evaluation of factors affecting biosynthesis ofDarabitol[J]. Food Science & Technology, 019,99:319- 327.
[15]Saha B C, SakakibaraY, Cotta M A. Production ofDarabitol by a newly isolated Zygosaccharomyces rouxii[J]. Journal of Industrial Microbiology and Biotechnol- ogy, 2007,34(7):519-523.
[16] Zhang Lili. Screening of high-yield D-arabinitol yeast strains and study of their fermentation conditions [D]. Jiangnan University, 2009.
[17]Nozaki H, Suzuki S, Tsuyoshi N, et al. Production ofDarabitol by Metschnikowia reukaufii AJ14787[J]. Bioscience, Biotechnology and Biochemistry, 2003,67(9):1923-1929.
[18]Cheng H, Lv J, Wang H, et al. Genetically engineered Pichiapastoris yeast for conversion of glucose to xylitol by a single-fermentation process[J]. Applied Mi- crobiology and Biotechnology, 2014,98(8):3539-3552.
[19]Zheng S, Jiang B, Zhang T, et al. Combined mutagenesis and metabolic regulation to enhanceDarabitol production from Candida parapsilosis[J]. Journal of Indus- trial Microbiology & Biotechnology, 2020,47(4):425-435.
[20] Wang L, Hui M, Yin Y, et al. Isolation and screening of a high-yield D-arabitol strain [J]. Journal of Food Safety and Quality Testing, 2014, 5(12):4018-4025.
[21] Toyoda T, Ohtaguchi K. Effect of temperature on Darabitol production from lactose by Kluyveromyces lactis [J]. Journal of Industrial Microbiology and Biotechnology, 2011, 38(9):1179-1185.
[22] Cai Li, Zhang Yang, Zhu Hongyang, et al. Isolation, screening and identification of a strain producing D-arabitol [J]. Food and Fermentation Industry, 2009, 35(01): 23-26.
[23] Saha B C, Cotta S M A. Production of d -arabitol by a newly isolated Zygosaccharomyces rouxii[J]. Journal of Industrial Microbiology & Biotechnology, 2007,34(7):519-523.
[24] Qian Weidong, Ning Xiaoxiao, Zhao Dezhi, et al. Improving the fermentation of Hansenula polymorpha to produce D-arabitol using a combination strategy [J]. Anhui Agricultural Science, 2014, 42(23): 7726-7728.
[25] Wang Gang, Tang Xiaofang, Zhang Guodong. Research on the conversion of glucose to arabitol by Hansenula anomala [J]. Shipin Gongye Ke-Ji, 2012(1):314-317.
[26] Zhang Lili, Liao Defang, Ding Chongyang, et al. Fermentation of glucose by Hansenula polymorpha to produce D-arabitol [J]. Industrial Microbiology, 2010, 40(04):47-52.
[27] Dou Yuan. Metabolic engineering of Pichia pastoris and biosynthesis of D-arabinitol [D]. Jiangsu University, 2022.
[28]Jagtap S S, Rao C V. Production ofDarabitol fromDxylose by the oleaginous yeast Rhodosporidium toruloides IFO0880[J]. Applied Microbiology and Biotech- nology, 2018,102(1):143-151.
[29]Yoshikawa J, HabeH, Morita T, et al. Production ofDarabitol from raw glycerol by Candida quercitrusa[J]. Applied Microbiology and Biotechnology, 2014,98(7):2947-2953.
[30] Wang Huilian, Yang Lianwan, Na Shumin, et al. Research on the production of glycerol and arabitol by yeast with high osmotic pressure resistance—III. Conditions for the production of glycerol by Zygosaccharomyces chevalieri Guill. 2.309 [J]. Acta Microbiologica Sinica, 1963(02):92-93.
[31] Toyoda T, Ohtaguchi K. Role of lactose on the production of Darabitol by Kluyveromyces lactis grown on lactose [J]. Applied Microbiology and Biotechnology, 2010, 87(2):691-701.
[32] Sun Wentao, Xu Hui, Liu Jianjun. Effect of additives on the fermentation of D-arabitol [J]. Food Science and Technology, 2013, 38(06): 12-16.
[33]Qi X, Zhang H, Magocha T A, et al. Improved xylitol production by expressing a novelDarabitol dehydrogenase from isolated Gluconobacter sp. JX-05 and co-bi- otransformation of whole cells[J]. Bioresour Technology, 2017,235:50-58.
[34]Ravikumar Y, Ponpandian LN, Zhang G, et al. Harnessing l-arabinose isomerase for biological production ofDtagatose: Recent advances and its applications[J]. Trends in Food Science & Technology, 2021,107:16-30.
[35]Koganti S, Ju L. Debaryomyces hansenii fermentation for arabitol production[J]. Biochemical Engineering Journal, 2013,79:112-119.
[36] Song Weibin, Lin Yanqing, Hu Haiyan, et al. Screening and identification of D-arabitol-producing strains and optimization of D-arabitol production conditions [J]. Acta Microbiologica Sinica, 2011, 51(03): 332-339.
[37]Song Weibin, Lin Yanqing, Hu Haiyan, et al. Isolation and identification of a novel Candida sp. H2 producingDarabitoland optimization ofDarabitol production [J]. Wei Sheng Wu Xue Bao, 2011,51(3):332-339.
[38]Sundaramoorthy B, Gummadi SN. Screening of new yeast Pichia manchurica for arabitol production[J]. Journal of Basic Microbiology , 2019,59(3):256-266.
[39]Kumdam H, Narayana M S, Gummadi S N. Production of ethanol and arabitol by Debaryomyces nepalensis: influence of process parameters [J]. AMB Express, 2013,3(1):23-28.
[40]Loman A A, Islam S, Ju L K. Production of arabitol from enzymatic hydrolysate of soybean flour by Debaryomyces hansenii fermentation[J]. Applied Microbiology and Biotechnology, 2018,102(2):641-653.
[41]Zhu H Y, Xu H, Dai X Y, et al. Production ofDarabitol by a newly isolated Ko- damaea ohmeri[J]. Bioprocess and Biosystems Engineering , 2010,33(5):565-571.
[42]Kumdam H, Murthy S N, Gummadi S N. Production of ethanol and arabitol by Debaryomyces nepalensis: influence of process parameters[J]. AMB Express, 2013,3(1):1-12.
[43]Ehrensberger AH,EllingRA, Wilson DK. Structure-guided engineering of xylitol dehydrogenase cosubstrate specificity[J]. Structure, 2006,14(3):567-575.
[44] Yuan Junhua. Construction of genetically engineered bacteria based on the 1,3-propanediol dehydrogenase of Clostridium butyricum and whole-cell biosynthesis of 1,3-propanediol [D]. Jiangsu University.
[45] Chen Gaoyun, Ye Kai, Tu Zhendong, et al. Research progress of xylitol dehydrogenase [J]. Brewing Science and Technology, 2011(5):4.
[46] Zhang Huanhuan. Cloning and expression of xylitol dehydrogenase and ethanol dehydrogenase genes from Gluconobacter oxydans and whole-cell biosynthesis of xylitol [D]. Jiangsu University, 2018.
[47]Sugiyama M, Suzuki S, Tonouchi N, et al. Cloning of the xylitol dehydrogenase gene from Gluconobacter oxydans and improved production of xylitol fromD arabitol[J]. Bioscience, Biotechnology and Biochemistry, 2003,67(3):584-591.
[48]Zhang J, Li S, Xu H, et al. Purification of xylitol dehydrogenase and improved production of xylitol by increasing XDH activity and NADH supply in Glucono- bacteroxydans[J]. Journal of Agricultural and Food Chemistry, 2013,61(11):2861- 2867.
[49]Qi X, Zhu J, Yun J, et al. Enhanced xylitol production: Expression of xylitol dehy- drogenase from Gluconobacter oxydans and mixed culture of resting cell[J]. Jour- nal of Bioscience and Bioengineering, 2016,122(3):257-262.
[50] He Peng. Molecular modification and mechanism analysis of enhanced catalytic efficiency of steroid P450 dihydroxylase CYP-cl3 [D]. Jiangnan University, 2022.
[51] Luo Yan. Cloning and co-expression of the arabitol dehydrogenase and xylitol dehydrogenase genes of Gluconobacter thailandicus D [D]. Jiangsu University, 2016.
[52] Zhang Yufei. Research on the co-production of 3-hydroxypropionic acid and 1,3-propanediol by Lactobacillus reuteri [D]. Jiangsu University, 2021.
[53] Yuan Jiao. Construction of a genetically engineered bacterium based on β-galactosidase and L-arabinose isomerase and biosynthesis of D-tagatose [D]. Jiangsu University, 2021.


 English
English French
French Spanish
Spanish Russian
Russian Korean
Korean Japanese
Japanese